1. Background
Many parts of the brain regions are considered as important sites for the molecular and cellular plasticity underlying addiction and exercise (1). These complex circuits include the hippocampus, cerebral cortex, ventral, dorsal striatum, mPFC, VTA area and amygdale (2). Prefrontal cortex has strong interactions with different parts of the brain and in many complex behavior involved with drug abuse. However, the anatomical and functional relationship of the mPFC with the hippocampus, LC, VTA area, and other parts of the brain suggest that the mPFC can have strong modulator effects on the mesocorticolimbic dopamine (DA) system, while itself being influenced by this system. Anatomically, the mPFC sends glutamatergic projections to both VTA and NAS (3) and receives dopaminergic input from the VTA, and glutamatergic input from other cortical areas and basal ganglia (4, 5). Many laboratories’ evidence for the involvement of the mPFC in mechanisms of addiction reported that the mPFC in the reward is not uniform. For example, the mPFC appears to be particularly important for the reward actions of cocaine, while it appears not to be important for the rewarding action of amphetamine (6). Also, the recent experiments propose, different subareas of the mPFC appear to be differently involved in the rewarding action of different drugs, such as, amphetamine and morphine (7). The available experimental evidence showed that some drugs can produce reward directly within the mPFC, and that some drugs, while not having direct rewarding effects within mPFC, depend on the function of the mPFC for the mediation of their rewarding effects (8).
Experimental and clinical findings propose that prolonged rhythmic exercise could activate the central opioid systems suggesting that exercise could be used to treat patients with addiction disorder (9). Both the exercise and drug addiction change the intracellular level of neurotransmitters. For example, extracellular levels of norepinephrine and glutamate decrease in the bed nucleus in chronic morphine-treated rats.
2. Objectives
Considering the anatomical and functional relationship between the mPFC area and the withdrawal symptoms of addiction, the aim of this study was to investigate the link between exercise, addiction, and the role of the mPFC area, leading to the development of new treatments for addiction.
3. Methods
3.1. Experimental Animals
This experimental study was performed on 50 male Wistar rats weighing approximately 250 - 300 gr. Because of avoiding the possible effects of the estrous cycle on test results, male rats were selected.
All rats were housed in 4-5 groups in each cage and they were kept under controlled conditions (temperature 20-24°C, relative humidity 40 to 70 % and light/darkness cycle 12/12 hours (light on at 8:00 a.m.).Food and water were available ad libitum. The measurements were always done during the first half of the light cycle. Rats were pre-tested to determine their treadmill running willingness and those rats which refused to run were excluded before the experiments started.
Rats were divided into five groups; each group consisted of 10 rats as follows:
1. Exercise: which received intraperitoneal (i.p.) 0.2 mL saline (9% NaCl) and participated in the treadmill exercise session (1 hour at speed of 17 m/min and with a slope of 15 degrees) for 10 days.
2. Morphine + Lesion: which had initially gone under stereotaxic operation and mPFC lesion, then received intraperitoneal morphine as follows: first 3 days 10 mg/ kg, next 3 days 20 mg/ kg and during last 3 days 40 mg/ kg and every day after receiving a dose of morphine. On the tenth day, the symptoms of addiction were evaluated.
3. Morphine + Exercise + Lesion: which had initially undergone stereotaxic operation and mPFC lesion, then received intraperitoneal morphine as follows: first 3 days 10 mg/ kg, next 3 days 20 mg/ kg and during last 3 days 40 mg/ kg and every day after receiving a dose of morphine was preceded by exercise. On the tenth day, the symptoms of addiction were evaluated.
4. Morphine: which received intraperitoneal morphine as follows: first 3 days 10mg/ kg, next 3 days 20 mg/ kg and during last 3 days 40 mg/ kg and on the tenth day, the symptoms of addiction were evaluated.
5. Morphine and Exercise: each morphine dose (see group 3) was preceded by exercise (see group 1). On the tenth day, the symptoms of addiction were evaluated.
3.2. Treadmill Running
Rats in the groups 1 (Exercise), 3 (Morphine + Exercise + Lesion) and 5 (Morphine and Exercise) were subjected to run at speed of 17m/min for 40 minutes daily (7 days a week), for 12 weeks at 0° inclination. To familiarize animals with the experimental set up, animals were left on the treadmill for 40 minutes once a day for 2 consecutive days without operation of the treadmill, then from the third day onward, the treadmill was switched on and the speed increased from 5 to 17 m/min and the duration increased from10 to 40 minutes over the course of 5 days. Electric shocks were used sparingly to motivate the animal to run. From week 2 onwards, after warm-up, speed and duration were kept constant at 17 m/min and 40 minutes per run. The non-runner groups were put on the treadmill without running for the same duration as the runners.
3.3. Stereotaxic Surgery and Lesion mPFC Area
For surgery, each rat initially with intraperitoneal chloral hydrate (0.5 mg/100 mL) was anaesthetized. Then, the animal’s head was fixed in a stereotaxic apparatus [Stealing Manufacturing Co. U.S.A] and after cleaning the point target on the surface of the skull and determining the Bregma and Lambda areas, by using Atlas (Paxinos Atlas) as the reference coordinates of the mPFC area (AP =3.2, DV = 2.5 and L = 0.6), the mPFC nucleus was destroyed. Finally, rats were transferred to separate cages and were subjected to a full care. After 3 days; they were used for continuing the project.
3.4. Withdrawal Syndrome Signs
The withdrawal syndrome was precipitated with an intraperitoneal injection of 2.0 mg/kg naloxone HCl, dissolved in saline. Withdrawal signs within 30 minutes after injection of naloxone in the last day were recorded. Abstinence signs were precipitated by naloxone in three experimental groups, and they consisted mainly of cycling, standing, licking, body lift and teeth chattering.
3.5. Statistical Analysis
Results are given as mean ± S.E.M. Data were analysed statistically using repeated measure of ANOVA (three-factor mixed ANOVA). A Tukeypost hoc test was done. Transforming data (square root) were considered when differences between the variances of the groups were significant. Probabilities less than 0.05 were considered significantly different.
4. Results
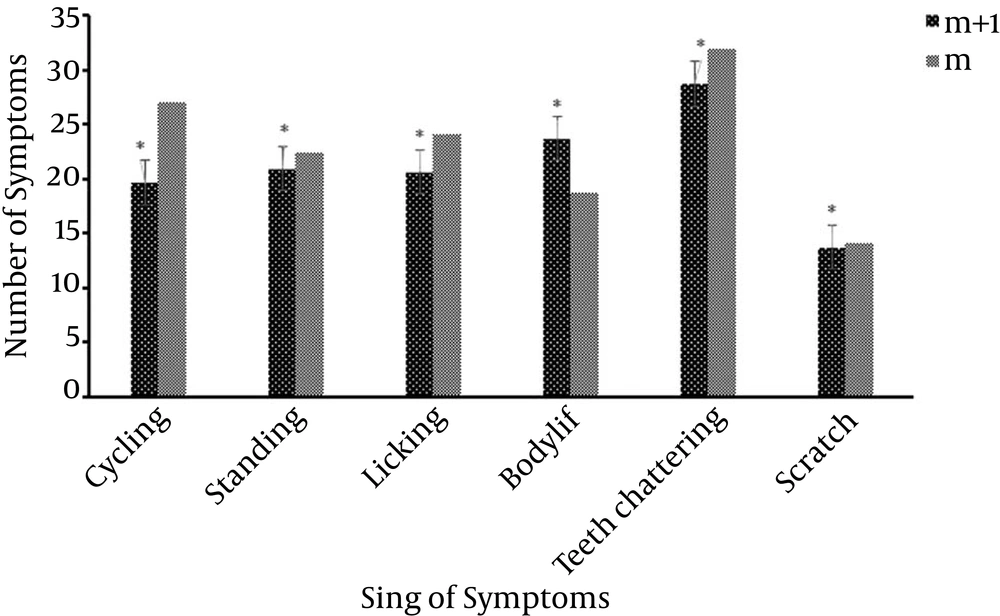
The survey results showed that mPFC area has a significant effect on addiction to opioid drugs. Most of the withdrawal symptoms in morphine + mPFC lesion groups (m + l groups) decreased. This reduction shows significant differences in compression with morphine groups (m group, Figure 1). This means, rats with Morphine + Lesion, which had an mPFC lesion, and then were treated with morphine, were able to relieve the withdrawal symptoms better than the Morphine groups (m groups). Therefore, mPFC area had an effect on weakening the trend to use morphine (Figure 2).
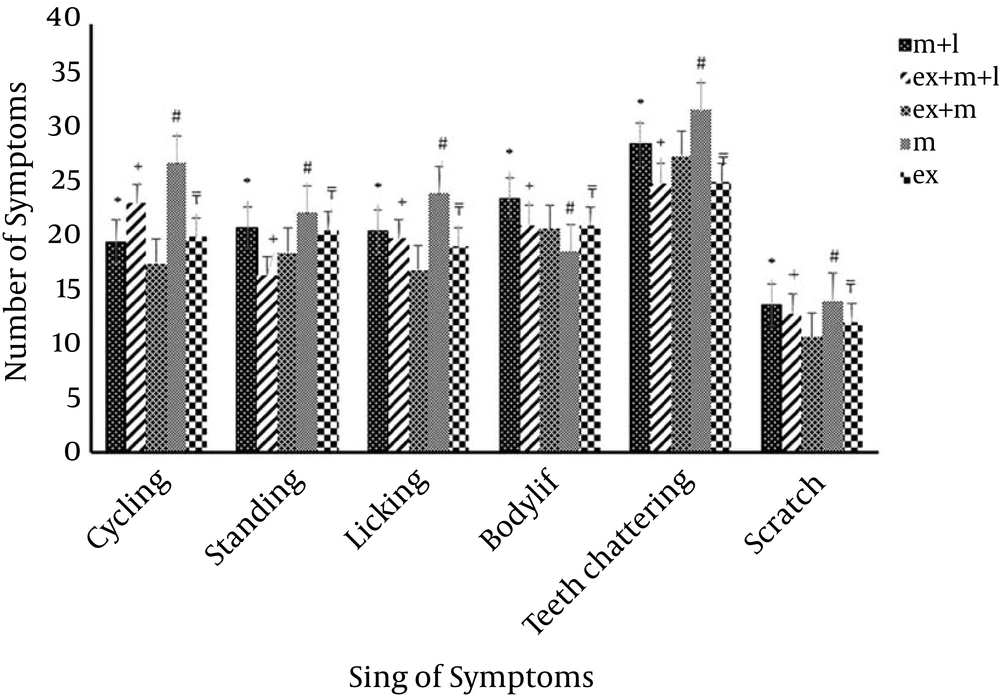
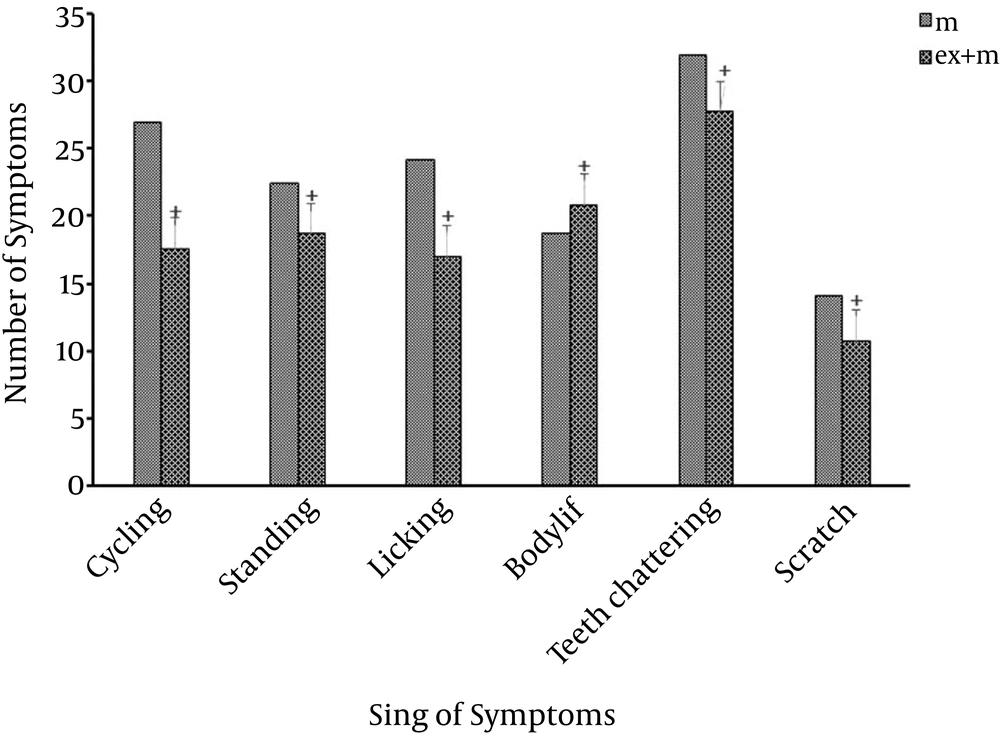
Exercise is an effective on the trend of using morphine and it reduced withdrawal symptoms significantly. (P < 0.05, Figure 3). Although, lesion of mPFC area reduced symptoms, as per the statistical analysis, this reduction did not show significant differences in compression with Morphine + Exercise + Lesion groups which is exercised, in most of sign of symptoms (P > 0.05) (Figure 2).
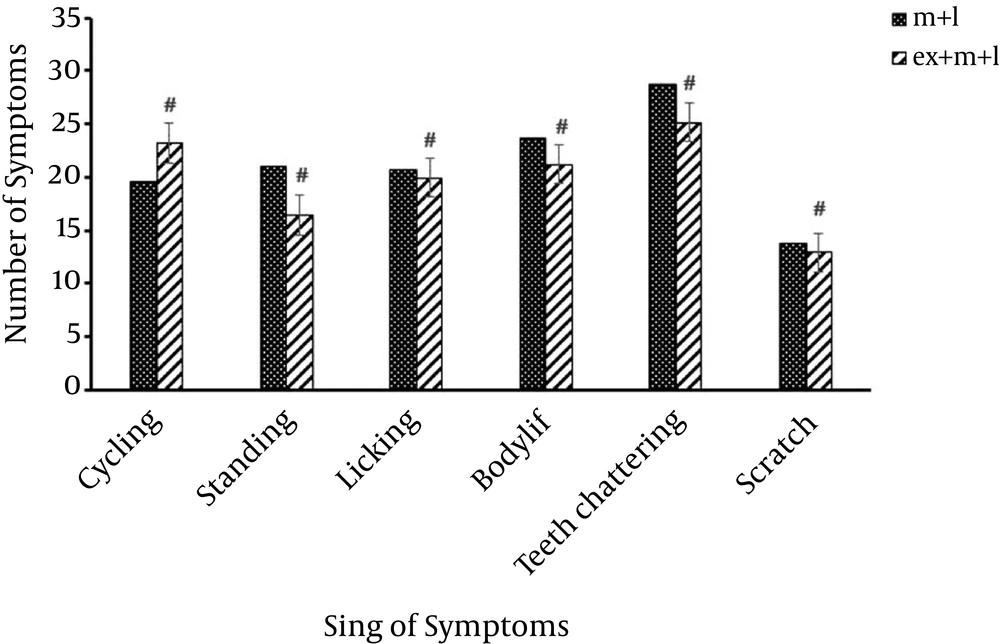
Further comparison between Morphine + Exercise + Lesion groups (m + ex + l) and Morphine + Lesion groups (m + l) indicated significant differences (#P < 0.05) in most symptoms. This means, rats treated with Morphine + Exercise + Lesion, which after having mPFC lesion, and then were treated with morphine and exercise, were able to relieve the withdrawal symptoms better than the Morphine + Lesion groups. Therefore, mPFC area had an effect on weakening the trend to use morphine (Figure 4).
Comparison the signs of withdrawal symptoms between the four groups (Morphine, Morphine + Exercise + Lesion, Morphine + Lesion groups, Morphine + Exercise) are shown in Figure 2. Reducing the tendency of using morphine in Morphine + Exercise + Lesion, who had received morphine-exercise and mPFC lesion, was certainly indicated in comparison with Morphine + Lesion groups (Figure 2). This means, the mPFC area has significant effects on morphine withdrawal signs which is processed in this area.
5. Discussion
The aim of this study was to evaluate the effects of short-term exercise on trends of use of morphine with an intact and lesion of mPFC area. The previous studies emphasize the role of physical activity in the promotion of learning and memory functions, and probably by cooperating with the mPFC area (10). The findings of this study showed that exercise decreased the tendency of using morphine and significantly enhances when compared with Morphine + Exercise + Lesion groups (Figure 2). Exercise has beneficial effects on decreasing the tendency to morphine and the rats’ treatments with morphine and exercise significantly reduced withdrawal symptoms (Figure 3). It has been also shown that certain drugs injected into the mPFC can produce conditioned place preference (CPP) or that lesions of the mPFC can disrupt the development of CPP (11).
The previous studies have shown that endogenous opioids are released by exercise (12) and endorphins that release after exercise increase the feeling of taking pleasure (13). Exercise also has encouragement effect on rats and this effect probably was mediated by opioid systems (14). All this evidence suggests that endogenous opioids levels are increased with physical activities (15). In another study, it was shown that addiction can be considered due to endorphin deficiency (16). Similarly, activating the endogenous opioid system after exercise in the present study has demonstrated a reduced desire of using morphine (Figure 2). In addition, the exercise will also effect the central dopaminergic and glutaminergic systems (16). It is clear that both exercise and drug addiction change the intracellular level of neurotransmitters. For example, extracellular levels of norepinephrine and glutamate decrease in the bed nucleus in chronic morphine-treated rats (17, 18). Morphine, like other drugs that have abuse liability, increases extracellular dopamine levels in the nucleus accumbens when administered systematically, or infused directly into mPFC (19-21). The importance of dopaminergic system in dependence on morphine and in response to physical activity has been shown in several studies (22, 23). The other study has shown that exercise changes the level of dopamine in brain and its metabolism is increased in certain regions of brain during exercise (23). Also, based on evidence observed in this study, exercise interferes in the dopamenargenic system, and changes the level of dopamine in different parts of the brain specially mPFC area. Therefore, many research efforts in this area have been made to determine the role of mPFC area in cognitive performances and addiction (24).
Briefly, the effect of short-term physical activity on withdrawal symptoms with, or without mPFC area in morphine-dependent rats was investigated, and it seems that mPFC area has the same effect on signs of addiction and other behaviors. Previous studies emphasized on the role of treadmill running in the improvement and promotion of learning and memory in morphine-treated rats. Memory deficit caused by morphine was also reversed by treadmill running, suggesting that physical activity enhanced learning and short term memory functions probably via the activation of release of some neurotransmitters in mPFC area (21).
It is likely that short-term exercise with opioid system and in the present of mPFC area reverse memory deficit by morphine and decreases the tendency of using morphine, and the results are another confirmation to this matter.