1. Background
Obesity is defined as abnormal or excessive fat accumulation that may impair health (1). Obesity is also associated with enhanced levels of reactive oxygen or nitrogen species (ROS, RNS), which accounts for obesity-induced diabetes, hypertension, dyslipidemia, atherosclerosis and cancer (2). Increased ROS production in obesity may be due to a reduction in the antioxidant defense system, which include enzymatic (e.g., superoxide dismutase (SOD) and glutathione peroxidase (GPX)) (3, 4) and nonenzymatic molecules (e.g., β-carotene and vitamin C) (5, 6). In addition, previous studies demonstrated that elevated leptin can increase intracellular ROS generation in obesity (7, 8). Therefore, elevated ROS and decreased antioxidant defense in obesity may stimulate cellular damage which is known as oxidative stress. It has further been suggested that oxidative stress is more elevated during exercise in obese populations (9, 10). Therefore, the use of antioxidant-rich diet to improve cellular damage caused by oxidative stress in obese people seems useful.
Recently, much attention has been focused on the herbal antioxidants to decrease oxidative stress during exercise in humans (11, 12). Green tea has been widely considered as a herbal antioxidant for alleviating oxidative stress (13). Green tea is produced from the tea plant (Camellia sinensis) that is grown in more than 30 countries, and is widely consumed as a beverage in the world (14). Green tea is a good source of polyphenol catechins which include (-)-epicatechin (EC), (-)-epigallocatechin (EGC), (-)-epicatechingallate (ECG), and (-)-epigallocatechingallate (EGCG) (15). EGCG is the most common catechin in green tea and exhibits the highest antioxidant activity (14). The antioxidant activity of green tea are achieved through direct and indirect mechanisms. Studies in vitro show that green tea catechins effectively scavenge reactive ROS such as superoxide and peroxyl radicals as well as RNS such as nitric oxide and peroxynitrite (16-18). In addition, a number of in vivo studies demonstrate that oral supplementation of green tea increases enzymatic antioxidants such as catalase, SOD, and GPX in animals (19-21). However, research on the effects of short-term intake of green tea on exercise-induced oxidative stress in obese populations are rare, but improvement in oxidative stress and antioxidant defense systems during intense exercise in athletes and untrained populations have been reported (11, 12, 22).
Furthermore, it was reported that obesity is associated with decreased skeletal muscle strength and function, impaired skeletal muscle mitochondrial respiratory function, higher ratios of type II to type I skeletal muscle fibers, as well as reduced antioxidant capacities, all of which contribute to increase in ROS production (23-26). Thus, obese people may be more susceptible to exercise-induced oxidative stress compared with nonobese individuals.
2. Objectives
Therefore, in order to reduce exercise-induced oxidative stress in obese populations, it was hypothesized that green tea supplementation would attenuate exercise-induced oxidative stress. To test this hypothesis, in a randomized, double-blind, placebo-controlled, crossover study; the effect of 2 week green tea extract supplementation on biomarkers of lipid peroxidation (8-iso PGF2α) and oxidative DNA damage (8-OHdG) after RE with 75% of one repetition maximum (1RM) were measured in obese men.
3. Methods
3.1. Subjects
Ten apparently healthy obese men volunteered for this study. The participants’ characteristics are presented in Table 1. Inclusion criteria were: nonsmokers, disease-free, not having used drugs or antioxidant supplements or any kind of food rich in polyphenolic compounds for the past 4 months, no participation in regular exercise training within the past 6 months, and a BMI above 30 kg.m-2. The study was approved by the Ethical Committee of the University of Kurdistan and all participants gave written informed consent to participate in the study.
| Group | Age, y | Weight, kg | Height, cm | BMI, kg.m-2 | WHR |
|---|---|---|---|---|---|
| Green tea extract | 37.12 ± 5.66 | 102.66 ± 14.43 | 177.25 ± 7.53 | 32.53 ± 2.52 | 0.99 ± 0.03 |
| Placebo | 37.12 ± 5.66 | 104.2 ± 13.56 | 177.25 ± 7.53 | 32.48 ± 2.39 | 1.00 ± 0.03 |
aValues are expressed as mean ± SD.
3.2. Experimental Procedures
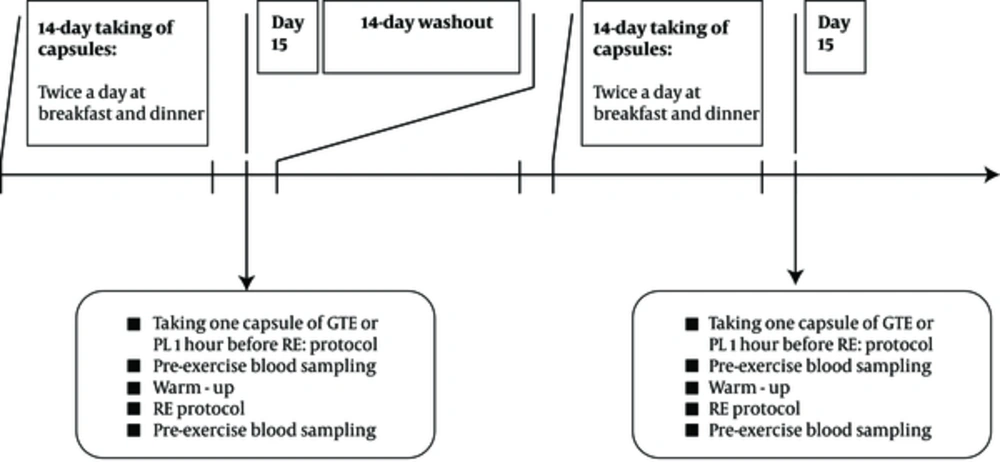
The obese men participated in a randomized, double-blind, placebo-controlled, crossover study consisting of two 14-day supplementations followed by a RE protocol and a one 14-day washout period (11, 12). One week before the two 14-day supplementation periods, participants visited the human performance laboratory for familiarization, filling the Par-Q health history questionnaire, and measurement of 1RM in bench press, lat pull down, biceps curl, leg flexion, leg extension and leg press according to previously described procedures (27). All participants were instructed not to change their normal diets and not to perform any physical activity during the course of the investigation. All participants completed three-day dietary records prior to RE at Saturday, Monday and Wednesday. The participants received a copy of their dietary record sheets and were asked to exactly follow the same food intake patterns (as recorded in their dietary record sheets) before the two RE protocols.
During two 14-day supplementation periods half of the subjects received GTE (2 capsules per day) and the other half PL (2 capsules per day), and vice versa. Both GTE and PL capsules were identical in shape, size and color. Participants consumed two capsules of GTE (250 mg GTE gelatin capsules, Olimp Labs, Poland) and PL (maltodextrin) twice a day at breakfast and dinner. One GTE capsule (55% EGCG) composed of 249 mg polyphenols which contain 200 mg catechins (137.5 mg EGCG). At the end of each two 14-day supplementation period, participants at the day 15 returned to the exercise physiology laboratory and took an additional capsule of GTE or PL at 1-hour before RE protocol which include 3 sets to exhaustion of bench press, lat pull down, biceps curl, leg flexion, leg extension and leg press with 75% of 1RM and 2 minutes rest between sets and exercises. Prior to RE protocols, all subjects performed warm-up, which consisted of 3 minutes running, 5 - 10 repetitions at 50% of perceived maximum and stretching period (Figure 1).
3.3. Blood Sampling and Laboratory Analysis
Blood samples were obtained form a forearm vein of each participant before (Pre) and immediately after (Post) RE. The serum was obtained by centrifugation at 3000 rpm for 10 minutes at 4ºC and frozen at -80ºC for later analysis. Oxidative DNA damage was determined using serum 8-OHdG concentration with a commercially available kit (ELISA kit, Cat.No:CK-E90285, HANGZHOU EASTBIOPHARM CO.,LTD). Lipid peroxidation was measured using serum 8-iso PGF2α concentration with a commercially available kit (ELISA kit, Cat.No:CK-E90228, HANGZHOU EASTBIOPHARM CO.,LTD). The average intra-assay coefficient of variation for 8-OHdG and 8-iso PGF2α was 3.8 and 6.1%, respectively. The sensitivity for 8-OHdG and 8-isoPGF2α was 0.25 ng/mL and 2.84 ng/L, respectively.
3.4. Statistical Analysis
Data are expressed as mean ± SD. Statistical evaluation was performed with SPSS (SPSS, Chicago, IL) for windows. The normality and variance of the data were performed using the Shapiro-Wilk and Levene tests, respectively. 8-OHdG and 8-isoPGF2α were analyzed using a 2 (groups) × 2 (times) repeated-measures analysis of variance (two-ways ANOVA). When interaction effects were significant (P ≤ 0.05), simple main effects were assessed using independent and paired t-test. Independent-sample t-tests were performed to determine possible group differences for the following variables; height, body mass, and dietary intake. The significance level was set at P < 0.05.
4. Results
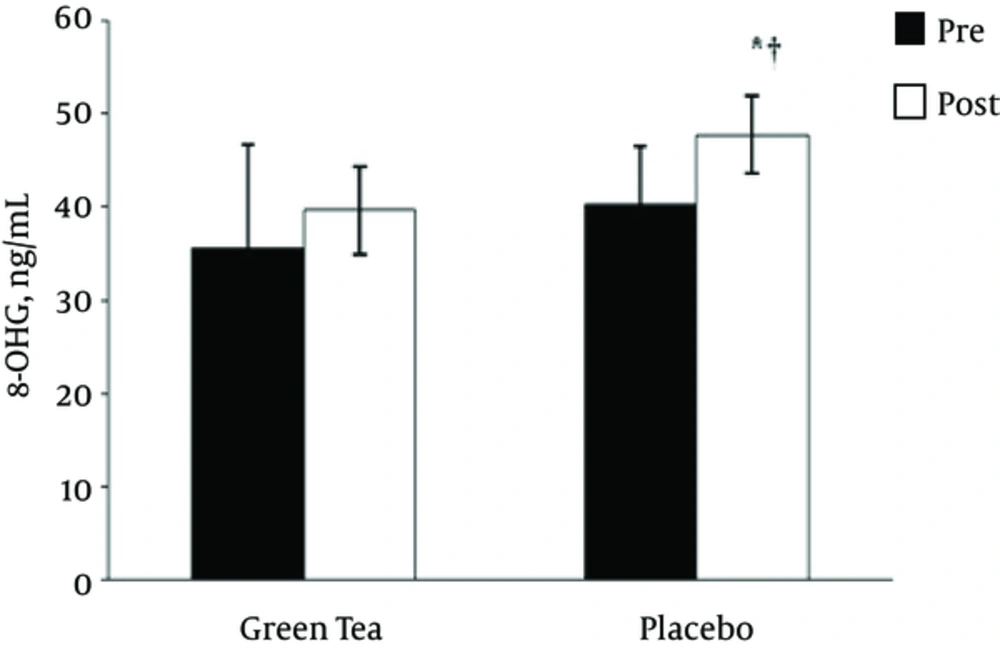
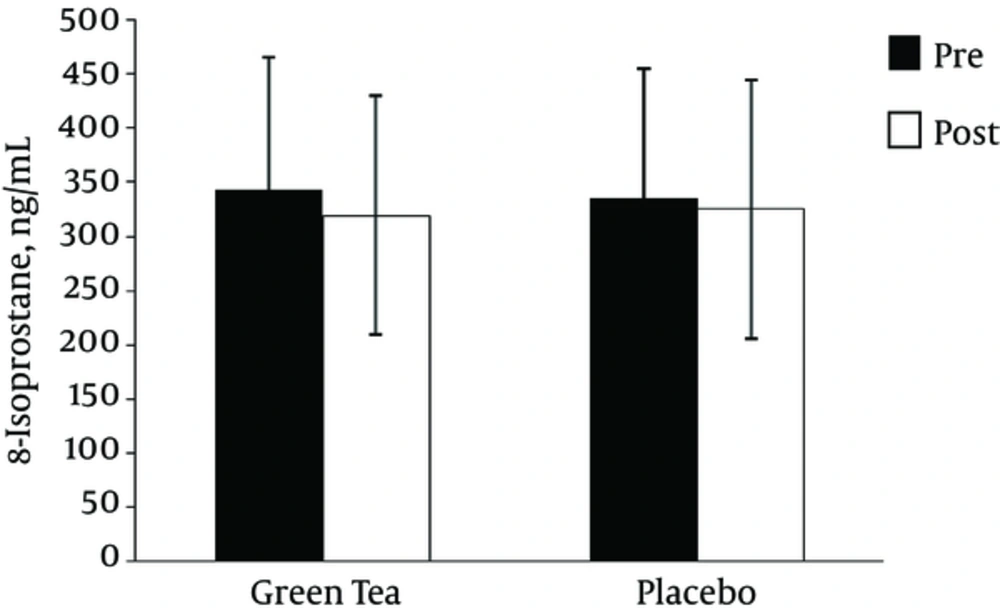
As depicted in Figure 2, the two-way time × group repeated-measures ANOVA for 8-OHdG indicated a main effect for group (F = 6.36, P = 0.024, observed power = 0.651), but no significant time effect (F = 2.64, p = 0.127, observed power = 0.32), or time × group interaction (F = 0.015, P = 0.905, observed power = 0.051). Independent-sample t-test revealed significant difference between the GTE and PL treatment at post RE (t = -3.625, P = 0.003), but there was no significant difference between the GTE and PL treatment at pre RE (t = -1.648, P = 012). In PL treatment 8-OHdG levels after RE was higher than pre RE (t = -4.25, P = 0.004). However, as depicted in Figure 3, the two-way time × group repeated-measures ANOVA for 8-isoPGF2α indicated no significant time effect (F =2.77, P = 0.118, observed power = 0.342), main effect for group (F = 0.001, P = 0.948, observed power = 0.050), and time × group interaction (F = 0.465, P = 0.507, observed power = 0.097).
| Variables | Mean ± SD | t | df | P Value | ||
|---|---|---|---|---|---|---|
| 8-OHdG, ng/mL | Pre | Green tea | 35.60 ± 11.16 | -1.648 | 14 | 0.12 |
| Placebo | 40.28 ± 6.19 | |||||
| Post | Green tea | 39.62 ± 4.74 | -3.625 | 14 | 0.003b | |
| Placebo | 47.75 ± 4.20 | |||||
| 8-iso PGF2α, ng/L | Pre | Green tea | 343.64 ± 121.59 | 0.137 | 14 | 0.89 |
| Placebo | 335.40 ± 118.73 | |||||
| Post | Green tea | 319.36 ± 110.09 | -0.102 | 14 | 0.92 | |
| Placebo | 325.22 ± 118.98 | |||||
aThese results are the mean ± standard deviation of green tea extract (pre and post) and placebo (pre and post) treatment.
bP < 0.05 when compared with the placebo treatment.
| Variables | Mean ± SD | t | df | Sig. | ||
|---|---|---|---|---|---|---|
| 8-OHdG, ng/mL | Green tea | Pre | 35.60 ± 11.16 | -1.34 | 7 | 0.22 |
| Post | 39.62 ± 4.74 | |||||
| Placebo | Pre | 40.28 ± 6.19 | -4.25 | 7 | 0.004b | |
| Post | 47.75 ± 4.20 | |||||
| 8-iso PGF2α, ng/L | Green tea | Pre | 343.64 ± 121.59 | 1.82 | 7 | 0.11 |
| Post | 319.36 ± 110.09 | |||||
| Placebo | Pre | 335.40 ± 118.73 | 0.643 | 7 | 0.54 | |
| Post | 325.22 ± 118.98 | |||||
aThese results are the mean ± standard deviation of green tea extract (pre and post) and placebo (pre and post) treatment.
bP < 0.05 relative to the pre resistance exercise (RE).
5. Discussion
RE was reported to increase the production of ROS in various tissues, which may lead to oxidative stress as measured by biomarkers of lipid peroxidation (27, 28), oxidative DNA damage (27, 29) and protein damage (30) in humans. In addition, recent studies have shown that RE-induced oxidative stress is more elevated in obese people than in non-obese people (9). Therefore, in this study the effects of 2-week green tea extract consumption on RE-induced oxidative stress were evaluated in obese men.
The main finding of our study was that the 2-week green tea extract consumption reduced RE-induce oxidative DNA damage in obese men. This suggests that GTE supplementation could effectively protect cells against attacks of ROS such as hydroxyl radicals, singlet oxygen, or photodynamic action which are responsible for the generation of 8-OHdG (31). Furthermore, our findings showed that RE with 75% of 1RM load performed after PL treatment caused a significant increase in oxidative DNA damage as measured by 8-OHdG concentration. Regarding the effects of RE on oxidative DNA damage, previous studies have demonstrated a significant increase in urinary 8-OHdG concentration 24 hours after a RE protocol (7 sets of 4 exercises using 60 - 90 1 repetition maximum) in the flat pyramid loading pattern (27), as well as a significant increase in urinary 8-OHdG at 3h post and 24 hours post RE with 75% of the 1RM and 90% of the 1RM (32). In addition, an increase in 8-OHdG of the quadriceps muscle was reported 24 hours after 200 eccentric actions with the knee extensors (33), whereas no change was reported in blood 8-OHdG after 30-minute dumbbell squats with 70% of 1RM (34). Overall, the findings of previous studies suggest that intense RE may cause oxidative DNA damage in trained and non-trained individuals. The precise mechanisms by which RE induced oxidative DNA damage is unknown, however, several possible mechanisms are reported as ways of generation of free radicals during RE; including (a) xanthine-xanthine oxidase pathway, (b) respiratory burst of neutrophils, (c) catecholamine auto oxidation, (d) local muscle ischemia-hypoxia, and (e) conversion of the weak superoxide to the strong hydroxyl radical by lactic acid (35).
It is well known that DNA oxidation is due to hydroxyl radicals and singlet oxygen attack on 2’-deoxyguanosine, resulting in a hydroxyl moiety replacing the hydrogen atom (36). Since oxidative DNA damage is involved in mutagenesis and induction of tumors (37), research has focused on strategies to reduce this damage at the cellular level. For this, previous studies have used antioxidant supplementation to ameliorate oxidative DNA damage after exercise, with some demonstrating decreased 8-OHdG levels after creatine monohydrate (27, 29), vitamin E supplementation (38), and others showing no protective effect after the intake of vegetable juice powder concentrate for two weeks (39).
Recently, researchers have focused on GTE consumption as a herbal antioxidant to decrease oxidative stress (11, 12, 40). Previous studies reported the positive effect of GTE on oxidative DNA damage in different cellular and animal models (41), but only few studies are available in vivo. Erba et al. (41) showed that consuming two cups of green tea (250 mg of total catechins) caused significant decrease in oxidative DNA damage induced by iron ions in healthy females. To our knowledge, there aren't studies regarding the effects of GTE on RE-induced oxidative DNA damage in the obese population. However, previous studies reported that supplementation with GTE prevent oxidative stress induced by repeated cycle sprint tests in male sprinters (22), 4 sets of RE in weight-trained men (12) and one set of bench press and back squat to exhaustion in healthy male (11).
The precise mechanism (s) by which, GTE reduces oxidative stress after RE are unclear. The blunting effect of GTE supplementation on oxidative DNA damage after RE may be attributed to antioxidant activity of green tea catechins especially EGCG that exhibits the highest antioxidant activity (14). A limitation of our study is that we did not measure total polyphenols and antioxidant activity in response to GTE supplementation after RE in obese men. However, other studies demonstrated significant increase in total polyphenol concentration at rest and at 5 minutes post RE in healthy males (11), as well as total antioxidant capacity and GSH after RE in athletes (12). It was demonstrated that green tea catechins exert antioxidant activity through direct mechanisms such as scavenging reactive ROS including superoxide and peroxyl radicals as well as RNS such as nitric oxide and peroxynitrite (16-18, 40), and indirect mechanisms through increases in enzymatic antioxidants such as catalase, SOD, and GPX in animals and humans (12, 19-21, 41).
In our study, lipid peroxidation was measured by 8-iso PGF2α which is reported as the most reliable marker of lipid peroxidation (42). No significant changes in lipid peroxidation were observed in GTE and PL treatment as a result of RE in obese men. These findings are inconsistent with previous studies showing significant reduction in the post exercise level of lipid hydroperoxide after 1 and 4 weeks of GTE (11, 12) as well as significant reduction in MDA level after 4 weeks of GTE supplementation (22).
Although obesity is a risk factor for decline in enzymatic antioxidant activity (9), significant increase in lipid peroxidation wasn’t observed in obese men participating in a single RE after 2 weeks of GTE supplementation. These findings are consistent with those of McAnulty et al. (43) who didn’t observe a significant increase in 8-iso PGF2α concentration after exhaustive RE, as well as, Mirzaei et al. (29) who didn’t observe significant increase in 8-iso PGF2α concentration after exhaustive exercise in athletes after a seven-day creatine monohydrate and placebo supplementation. However, Rietjens et al. (44) reported that the levels of 8-iso PGF2α in urine collected 24 hours after a single session of resistance exercise increased by 40% compared to the pre-exercise baseline values. Inconsistency in the results may be due to the difference in exercise protocols, aerobic vs. anaerobic exercise, the training status of participants, the time of sampling the type of samples and assays, and duration and doses of GTE supplementation.
A limitation of our study was that antioxidant activities such as superoxide dismutase, glutation peroxidase, catalase were not measured in response to 2-week GTE supplementation in obese men after RE. Therefore, in the future, it will be helpful to examine antioxidant activities in response to GTE supplementation in obese population.
5.1. Conclusion
As mentioned previously, obesity is associated with reduced antioxidant defense (9) and therefore obese people are more susceptible to oxidative stress especially in acute exercise. Therefore, supplementation with dietary antioxidants could enhance the antioxidant defense system of obese people, and in turn, may offer protection against oxidative stress induced by acute exercise. In summary, this study suggests that 2 weeks of GTE supplementation (400 mg catechins per day) may offer a protective effect against oxidative DNA damage induced by RE in obese men. Therefore, it is recommended that obese individuals use GTE supplementation before participating in RE to reduce oxidative DNA damage caused by RE.