1. Background
Soy protein serves as an important source of essential amino acids including the most important BCAA (branched chain amino acids) for long term maintenance in adult humans (1). Isolated soy protein (ISP) also contains naturally occurring compounds, including isoflavones and saponins that possess anti-oxidative, anti-inflammatory, immune-regulatory, anti-carcinogenic, and cardio-protective influences (2-7). Unaccustomed activities especially those with a high eccentric component like resistance activities, plyometric, long distance running and intermittent shuttle running (8-16) induce muscle damage, known in the literature as exercise induced muscle damage (EIMD) (17). Additionally, chemical changes associated with both inflammatory (18, 19) and oxidative stress processes have also been reported (19, 20) with muscle damage. Signs and symptoms following (EIMD) hamper muscle function and reduce performance on consequent days (21).
In an endeavor to reduce the detrimental effects of the above phenomenon, a number of interventions in the form of ergogenic aids (22), nutritional supplements (23) and antioxidant supplementation (24, 25) have been investigated showing varied success.
Due to controversy surrounding soy proteins, studies done in context to exercise and recovery (26, 27) on athletic population for a chronic period of time are limited. As a result, no clear consensus has arisen from the published literature regarding the efficacy of isolated soy proteins in attenuating muscle damage and enhancing recovery following a single bout of damaging exercise.
Given our knowledge of the effects of isolated soy protein and its naturally occurring compounds like isoflavones in stimulating protein synthesis and curtailing exercise induced free radical damage to the muscle. We aimed to investigate the influence of 4 weeks supplementation of isolated soy protein powder on exercise induced muscle damage after a single bout of eccentric activity in athletes. We specifically focused on biochemical markers including muscle damage, creatine kinase (CK), inflammation, highly sensitive c reactive protein (hs-cRP) and oxidative stress, myeloperoxidase (MPO) besides other indices of muscle performance and aerobic function. We hypothesized that ingestion of ISP would serve as a countermeasure to muscle damage, inflammation and oxidative stress induced by a single bout of eccentric protocol.
2. Objectives
To examine the effects of consumption of ISP for a chronic period (4 weeks) on muscle damage and recovery following a bout of damaging exercise.
3. Patients and Methods
3.1. Participants
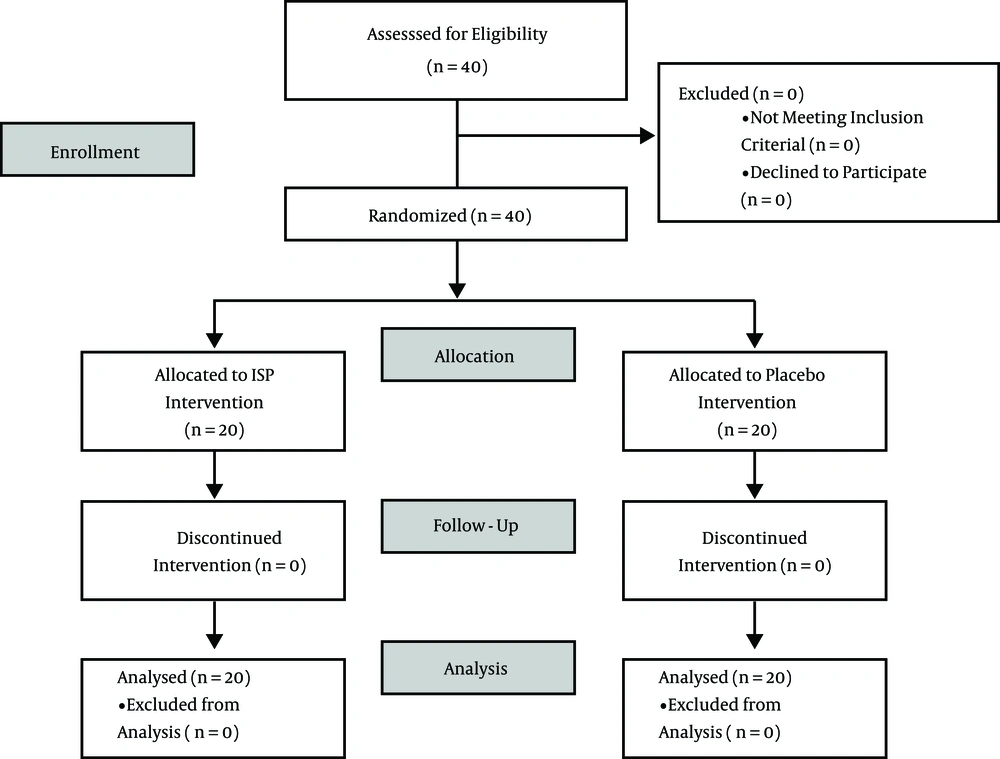
Forty trained male boxers and road cyclists volunteered to participate in this study (21). Based on the results of our study, considering mean (SD) change in Soy group 1.06 (0.5) and in Placebo group -0.01 (0.07) with alpha at 5% and sample size of 20 per group (n = 40 in total) the power of the study was calculated as 99.9%. Participants were mean (SD) 20.3 (1.49) years of age, 172.27 (6.42) cm tall and weighed 64.73 (5.44) kg; 20.03 (1.19) years of age, 168.57 (4.5) cm tall and weighed 61.74 (4.55) kg respectively. All participants who engaged themselves in specific, regular training for 30 hours a week during the competitive season were included in the study. Participants consuming any dietary supplements in the past 1 month were excluded from the study. The participants were briefed about the study protocol and provided a written, informed consent to participate in the study protocol. 20 participants each of both games were randomly and evenly allocated in a stratified, double blinded control manner among two groups (ISP and Placebo) and is summarized in Figure 1. All other investigators, study personnel, and subjects were blinded to the type of supplement used by the two groups during the study. Prior to the start of data collection, the trial was approved by Institutional Ethical Committee, Faculty of Sports Medicine and Physiotherapy, Guru Nanak Dev University.
3.2. Experimental Design
The experimental protocol followed a randomized, double blind (both the observer and the subjects were blinded), placebo controlled design (21). Randomization between the 2 groups was done using a computer generated random number tabulation by n query advisor version 7.
3.3. Procedure
The study employed a 4 week supplementation period with either ISP or placebo. Pre and post supplementation readings of the criterion variables, hs-cRP, CK, MPO, isometric muscle strength, maximum aerobic capacity heart rate and muscle soreness were obtained at baseline (Day 1), at 24 hours (Day 2) and at 48 hours (Day 3) following EIMD.
The study was carried in between the months of August 2013 to July 2014 at Human Performance Lab, department of sports medicine and physiotherapy, Guru Nanak Dev University, Amritsar. All testing sessions were performed in morning in a climatically-controlled laboratory under similar environmental conditions (temperature, 19.3 to 21.4°C; relative air humidity, 30 to 32%) (28). Participants were injury free and were off training (only maintaining a basic minimum of physical activity to prevent deconditioning) during the supplementation period, maintained regular dietary habits and avoided taking additional proteins and nutritional supplements (21).
3.4. Exercise Protocol
Participants performed a total of 100 drop-jumps from a height of 0.6 m. Upon landing, participants were encouraged to immediately jump vertically with maximal force. Five sets of 20 drop-jumps were performed with a 10 seconds interval between each jump and a 2 minutes rest between sets. This protocol has been previously shown to cause significant elevations in muscle damage markers (21, 29, 30).
3.5. Supplementation Protocol
Participants ingested 25 grams of ISP twice daily (26) or placebo (aspartame based artificial sweetener) mixed with ~300 mL of water (21) for 4 weeks. The ISP (NUSOWIN soy protein isolate powder, WINMEDICARE PVT. LTD., New Delhi, India) contained 21.1 grams of proteins and 21.1 mg of isoflavones genistein per serving (25g each). Artificial sweetener rather than a carbohydrate-based placebo was used to prevent a rise in insulin that may have altered protein metabolism (31). Before ingestion, supplement was tested for banned substances at national dope testing laboratory, New Delhi. The dosage ISP was based on manufacturer’s recommendations and previous supplementation research (26).
3.6. Biochemical Markers
Blood samples were drawn via standard venipuncture from the median cubital vein in the antecubital space of the forearm (32). Blood was drawn into heparin tubes and was spun at 3000 × g and the plasma was stored at -70̊C until analysis (27). Plasma was analyzed for CK and hs-cRP using assay kits from DiaSys diagnostic systems GmbH. (Holzeim, Germany). Plasma MPO was analyzed using ELISA kit from BioVendor, research and diagnostic products. (Brno, Czech republic).
3.7. Isometric Peak Torque Assessment
Isometric peak torque was assessed of both the quadriceps (extension) and the hamstrings (flexion) at the knee joint of both the limbs using HUR 5340 leg extension/curl, a computer controlled isoinertial dynamometer (33) following a warm up session.
3.8. Maximum Aerobic Capacity (VO2 max) Assessment
VO2 max was assessed using an open circuit breath by breath automated precalibrated portable gas analyzer MetaMax 3B (Cortex, Germany) integrated with a motorized treadmill h/p/cosmos mercury [cos 10198-01] (Germany). Prior to commencing the test each participant was told about the incremental exercise test protocol and continuous electronic heart rate monitoring throughout the test. Participants performed an incremental test to volitional exhaustion in accordance with the Bruce protocol (34). VO2 max was determined based on a plateau in VO2 consumption or participant reaching volitional fatigue (35).
3.9. Muscle Soreness
Participants performed and held a squat (90° knee angle) whilst they rated their perceived muscle soreness on a 0 - 10 cm visual analog scale (VAS) where 0 indicated no pain and 10 indicated severe pain (36).
3.10. Statistical Analysis
The data were analyzed using data analysis and statistical software STATA 12.1. All data are expressed as mean ± SD in each group. Within group effects were determined using paired t test and wilcoxon signed rank test. The normality was tested using Shapiro-Wilk and Shapiro Francia test. Statistical significance was set at P < 0.05 prior to analyses.
4. Results
All subjects completed the exercise protocols with all the dependent variables showing time changes demonstrating the protocol successfully induced muscle damage in both cyclists and boxers (Tables 1 and 3). Biochemical markers of inflammation (hs-cRP), muscle damage (CK), and oxidative stress (MPO), demonstrated an increase in the mean values following EIMD, both before and after supplementation in both the game players (Cyclists, Table 1a - 1c and boxers, Table 3a - 3c) whereas the degree of increase is less following soy consumption when compared to the placebo group (Table 1a - 1c and Table 3a - 3c). Additionally, a statistically significant (P < 0.01) group difference between the pre and post supplementation changes were found in both cyclists (Table 2a - 2c) and boxers (Table 4a - 4c).
No group difference was found in the pattern of change over time (from 24 hours to 48 hours post exercise values) in both pre and post supplementation period for these biomarkers in cyclists (Table 1a - 1c) and boxers (Table 3a - 3c), indicating muscle recovery. However, the efficacy of soy protein in enhancing the recovery process in context to inflammatory and muscle damage marker as depicted by a greater decrease in the mean values was found in boxers only (Table 3a and 3b).
Mean values of isometric peak torque at the knee joint (extension and flexion), measured for the right and left limb showed a similar pattern of decrease with no group difference 24 hours post exercise, followed by an improved muscle force in the subsequent day of recovery in all participants; cyclists (Table 1d - 1g) and boxers (Table 3d - 3g). The degree of decrease 24 hours post exercise was lower following consumption of soy in both the game players (Table 1d - 1g and Table 3d - 3g) demonstrating the effectiveness of the experimental supplement in attenuating the reduction in muscle force following damage.
Furthermore, statistically significant group difference (P < 0.05) between the pre and post supplementation changes were only observed among boxers (Table 4d - 4g). Correspondingly, soy consumption enhanced recovery as reflected by a greater degree of increase in the mean values obtained 48 hours following damaging exercise in both cyclists and boxers (Table 1d - 1g and Table 3d - 3g).
Groups did not differ in the pattern of change over time for VO2 max, measure of aerobic capacity. Soy supplementation attenuated the decrease in VO2 max in boxers only (Table 3h) as reflected in degree of change in the mean value measured at 24 hours post exercise.
Soy supplementation showed a positive ergogenic effect in context to heart rate response in all participants, both cyclists and boxers (Table 1i and Table 3i) when compared to the placebo group.
Muscle soreness peaked at 24 hours post exercise followed by a drop in the subsequent day of recovery in both the game players (Cyclists, Table 1j and boxers, Table 3j) with greater degree of decrease following soy consumption when compared to placebo.
| Groups | Pre Suppl (Day 1) | Pre Suppl (Day 2) | Pre Suppl (Day 3) | Post Suppl (Day 1) | Post Suppl (Day 2) | Post Suppl (Day 3) |
|---|---|---|---|---|---|---|
| a. hs-cRP (mg/dL) | ||||||
| Soy | 4.17 ± 0.4 | 10.02 ± 1.7 | 8.33 ± 1.4 | 3.62 ± 0.5 | 8.41 ± 1.4 | 7.01 ± 1.2 |
| Placebo | 3.94 ± 0.5 | 9.29 ± 1.1 | 7.79 ± 1.3 | 3.93 ± 0.6 | 9.30 ± 1.1 | 7.81 ± 1.3 |
| b. CK (IU/L) | ||||||
| Soy | 145.38 ± 8.8 | 461.24 ± 60.0 | 346.09 ± 63.3 | 132.05 ± 7.9 | 371.14 ± 70.3 | 259.86 ± 54.2 |
| Placebo | 140.8 ± 10.8 | 382.21 ± 37.6 | 335.57 ± 36.7 | 139.77 ± 10.4 | 382.75 ± 35.9 | 337.03 ± 36.9 |
| c. MPO (ng/L) | ||||||
| Soy | 8.80 ± 0.9 | 13.24 ± 1.9 | 11.03 ± 2.1 | 8.08 ± 0.8 | 11.19 ± 2.0 | 9.00 ± 1.7 |
| Placebo | 8.39 ± 0.9 | 13.43 ± 2.0 | 11.56 ± 1.8 | 8.37 ± 0.9 | 13.44 ± 2.0 | 11.56 ± 1.7 |
| d. ISOR EXT (Nm) | ||||||
| Soy | 188.70 ± 17.5 | 150.1 ± 17.7 | 165.3 ± 18.7 | 201.7 ± 17.3 | 165.1 ± 16.9 | 181.0 ± 16.7 |
| Placebo | 194.60 ± 17.1 | 172.40 ± 17.5 | 183.70 ± 17.4 | 194.7 ± 16.5 | 169.8 ± 14.9 | 184 ± 18.2 |
| e. ISOR FLEX (Nm) | ||||||
| Soy | 91.00 ± 10.6 | 52.80 ± 16.8 | 67.10 ± 14.9 | 103.2 ± 7.9 | 67.5 ± 14.9 | 82.4 ± 12.4 |
| Placebo | 92.50 ± 8.9 | 74.90 ± 7.6 | 86.20 ± 6.9 | 91.8 ± 10.2 | 75.3 ± 6.4 | 85.6 ± 7.2 |
| f. ISOL EXT (Nm) | ||||||
| Soy | 177.80 ± 11.1 | 143.60 ± 13.5 | 153.9 ± 11.6 | 189.8 ± 10.3 | 159.4 ± 13.1 | 169.9 ± 10.9 |
| Placebo | 178.50 ± 10.1 | 157.90 ± 9.9 | 172.10 ± 9.6 | 179.7 ± 9.6 | 156.2 ± 7.9 | 171.9 ± 10.4 |
| g. ISOL FLEX (Nm) | ||||||
| Soy | 89.20 ± 10.8 | 49.20 ± 16.9 | 62.80 ± 13.9 | 100.5 ± 8.8 | 62.8 ± 16.1 | 77.8 ± 12.9 |
| Placebo | 80.10 ± 6.9 | 66.30 ± 7.9 | 82.00 ± 8.3 | 80.3 ± 19.8 | 66.5 ± 8.8 | 81.5 ± 8.3 |
| h. VO2 (ml/kgminute-1) | ||||||
| Soy | 57.00 ± 4.0 | 54.90 ± 3.7 | 56.10 ± 3.6 | 58.7 ± 4.1 | 56.2 ± 3.7 | 57.6 ± 3.9 |
| Placebo | 56.60 ± 4.1 | 54.4 ± 4.4 | 55.60 ± 4.2 | 56.8 ± 4.3 | 54.4 ± 4.6 | 55.1 ± 4.8 |
| i. HR (beats/minute) | ||||||
| Soy | 182.80 ± 10.2 | 200.2 ± 10.4 | 192.60 ± 10.3 | 177.7 ± 10.1 | 192.80 ± 9.5 | 184.2 ± 10.8 |
| Placebo | 175.00 ± 8.3 | 193.6 ± 6.0 | 184.60 ± 4.6 | 175 ± 7.5 | 193.80 ± 6.2 | 182.00 ± 7.4 |
| j. VAS | ||||||
| Soy | - | 8.40 ± 0.7 | 7.10 ± 1.4 | - | 7.3 ± 0.7 | 5.8 ± 1.5 |
| Placebo | - | 8.20 ± 0.8 | 7.30 ± 0.8 | - | 8.00 ± 1.2 | 7.2 ± 1.0 |
Abbreviations: CK, creatine kinase; hs-cRP, highly sensitive C reactive protein; HR, heart rate; Iso R/L Ext/Flex, isometric right/left knee extension/ flexion; MPO, myeloperoxidase; Pre/Post Suppl, Pre and Post supplementation; VAS, visual analog scale; VO2, maximum oxygen consumption.
aValues are expressed as mean ± SD.
| Groups | A | B | Diff. AB (95% CI) | PAB-Value | C | D | Diff.CD (95% CI) | PCD-Value |
|---|---|---|---|---|---|---|---|---|
| a. hs-cRP (mg/d) | ||||||||
| Soy | 5.85 ± 1.7 | 4.79 ± 1.4 | 1.06 ± 0.5 (0.7 1.4) | < 0.001 | 1.68 ± 0.7 | 1.41 ± 0.9 | 0.28 ± 0.7 (0.2 0.8) | 0.26 |
| Placebo | 5.35 ± 0.7 | 5.37 ± 0.7 | -0.01 ± 0.07 (0.03 0.07) | 0.47 | 1.50 ± 0.8 | 1.49 ± 0.8 | 0.01 ± 0.06 (0.030.05) | 0.71 |
| b. CK (IU/L) | ||||||||
| Soy | 315.86 ± 59.5 | 239.09 ± 70.2 | 76.77 ± 19.1 (63.1 90.4) | < 0.001 | 115.15 ± 31.1 | 111.28 ± 42.7 | 3.87 ± 33.8 (20.3 28.1) | 0.72 |
| Placebo | 241.41 ± 35.7 | 242.98 ± 31.6 | -1.56 ± 7.1 (3.4 6.6) | 0.49 | 46.64 ± 21.8 | 45.72 ± 22.1 | 0.92 ± 4.9 (2.5 4.4) | 0.56 |
| c. MPO (ng/L) | ||||||||
| Soy | 4.44 ± 1.5 | 3.12 ± 1.6 | 1.32 ± 0.7 (0.8 1.8) | < 0.001 | 2.21 ± 0.6 | 2.19 ± 0.7 | 0.02 ± 1.1 (0.80 0.82) | 0.97 |
| Placebo | 5.04 ± 1.4 | 5.07 ± 1.4 | -0.03 ± 0.04 (0.0 0.06) | 0.05 | 1.87 ± 0.5 | 1.88 ± 0.5 | -0.01 ± 0.07 (0.04 0.05) | 0.79 |
| d. ISOR EXT (Nm) | ||||||||
| Soy | 38.6 ± 11.3 | 36.6 ± 13.8 | 2.0 ± 4.4 (1.1 5.1) | 0.18 | 15.2 ± 2.8 | 15.9 ± 4.1 | -0.7 ± 4.8 (2.7 4.1) | 0.66 |
| Placebo | 22.2 ± 6.0 | 24.9 ± 6.9 | -2.7 ± 9.6 (4.2 9.6) | 0.39 | 11.3 ± 2.7 | 14.2 ± 10.7 | -2.9 ± 9.1 (3.6 9.4) | 0.34 |
| e. ISOR FLEX (Nm) | ||||||||
| Soy | 38.2 ± 10.0 | 35.7 ± 9.7 | 2.5 ± 4.9 (1.0 6.0) | 0.14 | 14.3 ± 3.4 | 14.9 ± 5.0 | -0.6 ± 3.7 (2.1 3.2) | 0.62 |
| Placebo | 17.6 ± 4.9 | 16.5 ± 7.2 | 1.1 ± 3.8 (1.6 3.8) | 0.38 | 11.3 ± 3.0 | 10.3 ± 4.2 | 1.0 ± 2.1 (0.5 2.5) | 0.16 |
| f. ISOL EXT (Nm) | ||||||||
| Soy | 34.2 ± 9.7 | 30.4 ± 10.5 | 3.8 ± 4.8 (0.3 7.2) | <0.05 | 10.3 ± 11.0 | 10.5 ± 10.6 | -0.2 ± 5.1 (3.4 3.8) | 0.90 |
| Placebo | 20.6 ± 4.6 | 23.5 ± 6.8 | -2.9 ± 5.7 (1.2 6.9) | 0.14 | 14.2 ± 1.9 | 15.7 ± 6.7 | -1.5 ± 7.1 (3.6 6.6) | 0.52 |
| g. ISOL FLEX (Nm) | ||||||||
| Soy | 40 ± 11.9 | 37.7 ± 11 | 2.3 ± 4.7 (1.1 5.7) | 0.15 | 13.6 ± 4.1 | 15.0 ± 5.4 | -1.4 ± 3.6 (1.2 3.9) | 0.24 |
| Placebo | 13.8 ± 4.3 | 13.8 ± 16.4 | 0.0 ± 16.2, (-11.5 11.5) | 1.00 | 15.7 ± 3.6 | 15.0 ± 6 | -0.7 ± 4.9 (3.4 3.6) | <0.001 |
| h. VO2 (mL/kg.minute-1) | ||||||||
| Soy | 2.1 ± 0.8 | 2.5 ± 0.9 | -0.4 ± 0.7 (0.1 0.9) | 0.10 | 1.2 ± 0.63 | 1.4 ± 0.7 | -0.2 ± 0.6 (0.2 0.6) | 0.34 |
| Placebo | 2.2 ± 0.8 | 2.4 ± 0.5 | -0.2 ± 0.91 (0.4 0.8) | 0.50 | 1.2 ± 0.42 | 0.7 ± 1.1 | 0.5 ± 1.2 (0.4 1.4) | 0.24 |
| i. HR (beats/minute) | ||||||||
| Soy | 17.4 ± 3.2 | 15.1 ± 2.5 | 2.3 ± 2.7 (0.3 4.3) | 0.02 | 7.6 ± 2.0 | 8.6 ± 2.2 | -1.0 ± 2.9 (1.1 3.1) | 0.30 |
| Placebo | 18.6 ± 3.6 | 18.8 ± 4.5 | -0.2 ± 2.7 (1.8 2.2) | 0.82 | 9.0 ± 4.4 | 11.8 ± 4.7 | -2.8 ± 6.0 (1.5 7.1) | 0.17 |
| j. VAS | ||||||||
| Soy | - | - | - | - | 1.3 ± 0.9 | 1.5 ± 0.97 | -0.2 ± 0.42 (0.1 0.5) | 0.16 |
| Placebo | - | - | - | - | 0.9 ± 0.56 | 0.8 ± 0.63 | 0.1 ± 0.87 (0.5 0.7) | 0.72 |
aA, Degree of change in pre supplementation mean ± SD values from day 1 to day 2; B, degree of change in post supplementation mean ± SD values from day 1 to day 2; Diff.AB, difference between pre and post supplementation values; C, degree of change in pre supplementation mean ± SD values from day 2 to day 3; D, degree of change in post supplementation mean ± SD values from day 2 to day 3; Diff.CD, difference between pre and post supplementation values; hs-cRP, highly sensitive C reactive protein; CK-creatine kinase; MPO, myeloperoxidase; Iso R/L Ext/Flex, isometric right/left knee extension/ flexion; VO2, maximum oxygen consumption; HR, heart rate; VAS, visual analog scale; CI, confidence interval; SD, standard deviation; P, probability value; P ≤ 0.001(S), P ≤ 0.01(S), P ≤ 0.05(S).
| Groups | Pre Suppl (Day 1) | Pre Suppl (Day 2) | Pre Suppl (Day 3) | Post Suppl (Day 1) | Post Suppl (Day 2) | Post Suppl (Day 3) |
|---|---|---|---|---|---|---|
| a. hs-cRP (mg/dL) | ||||||
| Soy | 2.57 ± 0.7 | 6.17 ± 1.6 | 4.76 ± 1.6 | 2.38 ± 0.7 | 5.67 ± 1.6 | 3.70 ± 1.5 |
| Placebo | 2.39 ± 0.7 | 5.15 ± 1.8 | 3.74 ± 1.3 | 2.43 ± 0.7 | 5.16 ± 1.8 | 3.79 ± 1.3 |
| b. CK (IU/L) | ||||||
| Soy | 117.87 ± 11.9 | 354.05 ± 51.3 | 283.44 ± 46.8 | 105.96 ± 9.9 | 296.61 ± 51.3 | 221.21 ± 40.5 |
| Placebo | 117.86 ± 15.3 | 347.25 ± 42.2 | 286.67 ± 62.7 | 119.2 ± 16.2 | 349.31 ± 41.2 | 287.14 ± 61.1 |
| c. MPO (ng/L) | ||||||
| Soy | 6.11 ± 1.1 | 9.73 ± 2.1 | 7.99 ± 1.5 | 5.79 ± 1.1 | 8.27 ± 1.8 | 6.65 ± 1.3 |
| Placebo | 5.74 ± 1.0 | 8.88 ± 1.8 | 7.45 ± 1.4 | 5.74 ± 0.9 | 8.83 ± 1.8 | 7.52 ± 1.4 |
| d. ISOR EXT (Nm) | ||||||
| Soy | 190.4 ± 21.5 | 144.8 ± 16.7 | 157.2 ± 17.5 | 197.6 ± 21.2 | 159.2 ± 15.9 | 172.8 ± 16.3 |
| Placebo | 198.6 ± 20.5 | 174.8 ± 17.8 | 184.6 ± 16.3 | 198.1 ± 20.9 | 175 ± 18.4 | 184.7 ± 16.3 |
| e. ISOR FLEX (Nm) | ||||||
| Soy | 92.1 ± 13.1 | 44.3 ± 13.4 | 59.1 ± 12.2 | 99.4 ± 11.8 | 58.9 ± 12.9 | 77.1 ± 12.7 |
| Placebo | 92.1 ± 11.6 | 71.1 ± 9.9 | 78.7 ± 10.9 | 92.2 ± 11.2 | 68.5 ± 10.6 | 75.8 ± 10.8 |
| f. ISOL EXT (Nm) | ||||||
| Soy | 171.8 ± 10.8 | 138.3 ± 15.1 | 149.0 ± 15 | 182.1 ± 10.3 | 153.5 ± 16.1 | 164.8 ± 16.1 |
| Placebo | 175.4 ± 13.4 | 157.4 ± 15.3 | 173.9 ± 21.5 | 175.5 ± 13.4 | 143.5 ± 33.7 | 181.1 ± 16.6 |
| g. ISOL FLEX (Nm) | ||||||
| Soy | 87.9 ± 9.9 | 44.5 ± 14.3 | 59.4 ± 11.5 | 94.6 ± 9.6 | 58.4 ± 13.7 | 74.5 ± 10.6 |
| Placebo | 86.3 ± 12.3 | 63.8 ± 11.4 | 76.0 ± 11.4 | 86.8 ± 13.4 | 63.4 ± 11.0 | 78.4 ± 10.4 |
| h. VO2 (ml/kgminute-1) | ||||||
| Soy | 51.8 ± 3.6 | 48.8 ± 3.7 | 50.2 ± 4.1 | 53.50 ± 2.9 | 51.2 ± 3.6 | 52.0 ± 3.4 |
| Placebo | 50.6 ± 2.8 | 48.4 ± 2.5 | 49.8 ± 2.8 | 51.00 ± 3.1 | 48.6 ± 3.4 | 49.5 ± 3.1 |
| i. HR (beats/minute) | ||||||
| Soy | 172.2 ± 12.7 | 190.4 ± 11.2 | 185.1 ± 6.7 | 167.7 ± 10.9 | 181.6 ± 12.2 | 173.7 ± 9.9 |
| Placebo | 176.4 ± 12.1 | 192.7 ± 11.4 | 182.4 ± 10.5 | 173.4 ± 11.3 | 189.7 ± 11.2 | 179.7 ± 7.7 |
| j. VAS | ||||||
| Soy | - | 6.9 ± 0.9 | 4.7 ± 0.9 | - | 5.5 ± 0.7 | 2.9 ± 1.2 |
| Placebo | - | 4.9 ± 0.9 | 4.1 ± 0.7 | - | 5.1 ± 0.9 | 4.3 ± 1.3 |
aPre/Post Suppl, Pre and Post supplementation; hs-cRP, highly sensitive C reactive protein; CK, creatine kinase; MPO, myeloperoxidase; Iso R/L Ext/Flex, isometric right/left knee extension/flexion;VO2, maximum oxygen consumption; HR, heart rate; VAS, visual analog scale.
bValues are expressed as mean ± SD.
| Groups | A | B | Diff.AB (95% CI) | PAB-Value | C | D | Diff.CD (95% CI) | PCD-Value |
|---|---|---|---|---|---|---|---|---|
| a. hs-cRP (mg/dL) | ||||||||
| Soy | 3.60 ± 1.15 | 3.30 ± 1.23 | 0.3 ± 0.16 (0.19 0.42) | < 0.001 | 1.41 ± 0.36 | 1.97 ± 0.48 | -0.56 ± 0.24 (0.72 0.38) | < 0.001 |
| Placebo | 2.76 ± 1.19 | 2.73 ± 1.18 | 0.03 ± 0.07 (0.02 0.08) | 0.20 | 1.41 ± 0.59 | 1.37 ± 0.57 | 0.04 ± 0.09 (0.02 01) | 0.16 |
| b. CK (IU/L) | ||||||||
| Soy | 236.18 ± 48.18 | 190.65 ± 50.11 | 45.53 ± 17.72 (32.85 58.21) | < 0.001 | 70.61 ± 32.76 | 75.4 ± 43.3 | -4.8 ± 27.9 (15.1 24.7) | 0.6 |
| Placebo | 229.39 ± 35.08 | 230.11 ± 33.04 | -0.71 ± 5.29 (3.07 4.51) | 0.67 | 60.58 ± 42.69 | 62.17 ± 43.44 | -1.59 ± 7.46 (-6.9 3.7) | 0.51 |
| c. MPO (ng/L) | ||||||||
| Soy | 3.63 ± 1.27 | 2.48 ± 0.95 | 1.15 ± 0.4 (0.87 1.43) | < 0.001 | 1.75 ± 0.86 | 1.62 ± 0.80 | 0.13 ± 0.29 (-0.08 0.3) | 0.20 |
| Placebo | 3.14 ± 0.85 | 3.09 ± 0.87 | 0.05 ± 0.2 (0.09 0.20) | 0.42 | 1.44 ± 0.43 | 1.31 ± 0.56 | 0.13 ± 0.25 (-0.05 0.3) | 0.12 |
| d. ISOR EXT (Nm) | ||||||||
| Soy | 45.6 ± 11.57 | 38.4 ± 11.25 | 7.2 ± 2.25 | < 0.001 | 12.4 ± 2.59 | 13.6 ± 5.27 | -1.2 ± 4.37 (4.3 1.9) | 0.40 |
| Placebo | 23.8 ± 4.92 | 23.1 ± 5.3 | 0.7 ± 6.95 (-4.27 5.67) | 0.75 | 9.8 ± 3.29 | 9.7 ± 8.2 | 0.1 ± 8.67(6.1 6.3) | 0.97 |
| e. ISOR FLEX (Nm) | ||||||||
| Soy | 47.8 ± 11.69 | 40.5 ± 11.75 | 7.3 ± 3.92 (4.49 10.10) | < 0.001 | 14.8 ± 5.30 | 18.2 ± 4.82 | -3.4 ± 2.37 (1.7 5.09) | 0.001 |
| Placebo | 21 ± 1.43 | 23.7 ± 2.36 | -2.7 ± 4.64 (-6.02 0.62) | 0.09 | 7.6 ± 2.54 | 7.3 ± 2.95 | 0.3 ± 4.16 (2.6 3.2) | 0.82 |
| f. ISOL EXT (Nm) | ||||||||
| Soy | 33.5 ± 10.01 | 28.6 ± 10.24 | 4.9 ± 3.60 (2.3 7.4) | < 0.01 | 10.7 ± 2.11 | 11.3 ± 4.69 | -0.6 ± 4.97 (4.1 3) | 0.71 |
| Placebo | 18 ± 7.35 | 32 ± 25.80 | -14 ± 27.73 (-33.8 5.8) | 0.14 | 16.5 ± 9.98 | 37.6 ± 28.21 | -21.1 ± 30.39 (42.7 0.5) | 0.05 |
| g. ISOL FLEX (Nm) | ||||||||
| Soy | 43.4 ± 10.46 | 36.2 ± 10.36 | 7.2 ± 1.62 (6.04 8.3) | < 0.001 | 14.9 ± 6.76 | 16.1 ± 6.60 | -1.2 ± 2.44 (2.9 0.5) | 0.15 |
| Placebo | 22.5 ± 3.4 | 23.4 ± 3.13 | -0.9 ± 3.44 (-3.3 1.5) | 0.43 | 12.2 ± 2.89 | 15 ± 5.47 | -2.8 ± 5.22 (6.5 0.93) | 0.12 |
| h. VO2 (mL/kg.minute-1) | ||||||||
| Soy | 3 ± 0.47 | 2.3 ± 1.3 | 0.7 ± 1.5 (-0.37 1.8) | 0.20 | 1.4 ± 0.79 (0.8) | 0.8 ± 0.70 | 0.6 ± 1.17 (0.2 1.4) | 0.14 |
| Placebo | 2.2 ± 0.63 | 2.4 ± 0.51 | -0.2 ± 0.92 (0.85 0.45) | 0.50 | 1.4 ± 0.52 | 0.9 ± 0.74 | 0.5 ± 0.85 (0.1 1.1) | 0.09 |
| i. HR (beats/minute) | ||||||||
| Soy | 18.2 ± 4.54 | 13.9 ± 7.0 | 4.3 ± 6.4 (0.3 8.9) | 0.06 | 5.3 ± 11.8 | 7.9 ± 5.9 | -2.6 ± 9.5 (-9.4 4.2) | 0.40 |
| Placebo | 16.3 ± 3.4 | 16.3 ± 2.54 | 0.0 ± 5.2 (-3.7 3.7) | 1.00 | 10.3 ± 4.08 | 10 ± 5.1 | 0.3 ± 5.7 (-3.8 4.4) | 0.87 |
| j. VAS | ||||||||
| Soy | - | - | - | - | 2.2 ± 1.39 | 2.6 ± 1.26 | -0.4 ± 0.69 (0.1 0.9) | < 0.001 |
| Placebo | - | - | - | - | 0.8 ± 0.42 | 0.8 ± 1.1 | -0.2 ± 1.23 (1.0 0.6) | < 0.01 |
aA, Degree of change in pre supplementation mean ± SD values from day 1 to day 2; B, degree of change in post supplementation mean ± SD values from day 1 to day 2; Diff.AB, difference between pre and post supplementation values; C, degree of change in pre supplementation mean ± SD values from day 2 to day 3; D, degree of change in post supplementation mean ± SD values from day 2 to day 3; Diff.CD, difference between pre and post supplementation values; hs-cRP, highly sensitive C reactive protein; CK-creatine kinase; MPO, myeloperoxidase; Iso R/L Ext/Flex, isometric right/left knee extension/flexion; VO2, maximum oxygen consumption; HR, heart rate; VAS, visual analog scale; CI, confidence interval; SD, standard deviation; P, probability value; P ≤ 0.001(S); P ≤ 0.01(S); P ≤ 0.05(S).
| Variables | Soy | Placebo |
|---|---|---|
| Age, y | 20.4 ± 1.4 | 19.4 ± 0.9 |
| Height, m | 1.68 ± 0.06 | 1.69 ± 0.05 |
| Weight, kg | 63.7 ± 6.08 | 60.9 ± 3.3 |
| BMI | 22.2 ± 1.05 | 21.3 ± 1.06 |
| hs-cRP, mg/d | 4.17 ± 0.4 | 3.94 ± 0.5 |
| CK, IU/L | 145.38 ± 8.8 | 140.8 ± 10.8 |
| MPO, ng/L | 8.80 ± 0.9 | 8.39 ± 0.9 |
| ISOR EXT, Nm | 188.70 ± 17.5 | 194.60 ± 17.1 |
| ISOR FLEX, Nm | 91.00 ± 10.6 | 92.50 ± 8.9 |
| ISOL EXT, Nm | 177.80 ± 11.1 | 178.50 ± 10.1 |
| ISOL FLEX, Nm | 89.20 ± 10.8 | 80.10 ± 6.9 |
| VO2, mL/kg.minute-1 | 57.00 ± 4.0 | 56.60 ± 4.1 |
| HR, beats/minute | 182.80 ± 10.2 | 175.00 ± 8.3 |
| VAS | 8.40 ± 0.7 | 8.20 ± 0.8 |
Abbreviations: hs-cRP, highly sensitive C reactive protein; CK, creatine kinase; MPO, myeloperoxidase; Iso R/L Ext/Flex, isometric right/left knee extension/ flexion; VO2, maximum oxygen consumption; HR, heart rate; VAS, visual analog scale.
aValues are expressed as mean ± SD.
| Variables | Soy | Placebo |
|---|---|---|
| Age, y | 19.9 ± 1.1 | 21.0 ± 1.56 |
| Height, m | 1.73 ± 0.08 | 2.92 ± 0.2 |
| Weight, kg | 64.7 ± 6.4 | 65.6 ± 5.6 |
| BMI | 21.5 ± 1.1 | 22.5 ± 1.1 |
| hs-cRP, mg/d | 2.57 ± 0.7 | 2.39 ± 0.7 |
| CK, IU/L | 117.87 ± 11.9 | 117.86 ± 15.3 |
| MPO, ng/L | 6.11 ± 1.1 | 5.74 ± 1.0 |
| ISOR EXT, Nm | 190.4 ± 21.5 | 198.6 ± 20.5 |
| ISOR FLEX, Nm | 92.1 ± 13.1 | 92.1 ± 11.6 |
| ISOL EXT, Nm | 171.8 ± 10.8 | 175.4 ± 13.4 |
| ISOL FLEX, Nm | 87.9 ± 9.9 | 86.3 ± 12.3 |
| VO2, mL/kgminute-1 | 51.8 ± 3.6 | 50.6 ± 2.8 |
| HR, beats/minute | 172.2 ± 12.7 | 176.4 ± 12.1 |
| VAS | 6.9 ± 0.9 | 4.9 ± 0.9 |
Abbreviations: hs-cRP, highly sensitive C reactive protein; CK, creatine kinase; MPO, myeloperoxidase; Iso R/L Ext/Flex, isometric right/left knee extension/flexion;VO2, maximum oxygen consumption; HR, heart rate; VAS, visual analog scale.
aValues are expressed as mean ± SD.
5. Discussion
To the best of our knowledge, this is the first well controlled experimental trial to observe the efficacy of a vegetable based protein like soy in reducing the biochemical markers of inflammation, muscle damage and oxidative stress along with improvement in indices of muscle performance and aerobic function following a single bout of damaging exercise in athletes. We found that isolated soy protein consumption for 4 weeks led to decreased muscle damage and enhanced recovery as reflected by an increased degree of changes in the mean values of all the dependent variables over time when compared to placebo.
5.1. Effect of Isolated Soy Protein on Biochemical Markers
Isolated Soy protein was effective in reducing muscle damage, inflammation and oxidative stress in both game players as evidenced by difference in the degree of changes between pre and post supplementation mean values of hs-cRP, CK and MPO respectively. In support of our findings, Rossi et al. found that consumption of isolated soy protein for a period of 3 weeks caused a decrease in the levels of CK and MPO following exhaustive exercise protocol. These results support the antioxidant properties of soy protein as oxidant stress is believed to be a major contributor to muscle injury during exercise (26). However, it is not clear yet whether this effect was due to a reduction in neutrophil number, or due to a reduction in activation of secretory activities of individual neutrophils. The latter action of the soy isoflavone genistein has been observed in phagocytes in vitro (37).
5.2. Effect of Isolated Soy Protein on Isometric Muscle Strength and Aerobic Function
We found a decrease in the reduction of isometric muscle strength and a faster recovery of flexors and extensors of both the limbs with soy protein supplementation. Additionally, only in boxers, the group difference between the pre and post supplementation changes reached statistical significance (P < 0.05). There is little information in exercise literature with respect to effects of soy protein consumption on change in isometric muscle strength after damaging bout of exercise. The possible rationale behind the decrease in reduction of muscle strength following supplementation post exercise induced muscle damage may be attributed to the regulatory function of isolated soy protein on muscle protein metabolism (38, 39).
A decrease of maximum oxygen consumption (VO2 max), and an increase of HR response following exercise induced muscle damage was also observed. This was followed by significant improvement in VO2 max. It has been assumed that an increase in lactate contributes to an augmented ventilatory response following EIMD (40). Thus, the possible mechanism surrounding the improvement seems to be related to an improved lactate threshold as suggested by Matsumoto et al. (41).
The process of muscle soreness has been attributed to the establishment of an acute inflammatory response, resulting from metabolic, mechanical and oxidative stress (42). The VAS scores, measure for muscle soreness showed a reduction post supplementation with isolated soy protein suggesting the effectiveness of supplement in decreasing the subjective symptoms of inflammation i.e pain, on subsequent days of recovery following EIMD. To understand the plausible mechanism behind the decrease in muscle soreness warrants the need of additional time course and dose response studies with soy proteins.
The study is not without limitations. The diet of the participants was not controlled during this study and individual differences in the protein consumption could have affected the bioavailability of amino acids at the substrate level, hence affecting protein turnover and eventually influencing the outcomes of the study.
5.3. Conclusion
Taken in total, we found that isolated soy proteins are essential for muscle support following eccentrically induced muscle damage as they are useful sources of essential amino acids as well as antioxidants. Vegetable based proteins like soy, due to their similar digestibility and absorption kinetics to popular animal based supplements like whey can serve as a cost effective and affordable alternative for all athletes especially vegan athletes across the globe. Finally, additional research on soy proteins particularly in context to exercise induced muscle damage on highly trained individuals is needed to understand the molecular kinetics, changes in the physiological responses over a period of time post exercise when compared to more popular animal based proteins like whey, having similar digestibility and absorption kinetics.