INTRODUCTION
The effects of chronic stress and physical activity on cognitive functions have separately been the hot topics in neuroscience. Impairment of learning and memory processes has been demonstrated by many studies using different stressors [1]. Exposure to chronic restraint stress in rats and psychosocial stress in humans has been shown to alter cognitive functions and has been linked to the pathophysiology of disorders [2], hence stress can increase vulnerability to neuronal cell death [3]. In this way, several studies have investigated possible ways for protection of their deleterious effects [4]. Physical activity is one of the strategies that are suggested to enhance cognitive functions [5].
The positive effects of exercise on many physiological systems, including the central nervous system, are well-established [6]. Previous human and animal studies indicated that exercise can facilitate retention of memory in various hippocampal-dependent tasks including the passive avoidance [6, 7], active avoidance [8], Morris water maze [9, 10], radial arm maze [11], radial arm water maze [12, 13] and object recognition [14]. Hence exercise has beneficial and neuroprotective effects on brain cognitive functions, and it leads to changes at neuronal activity, synaptic structure and the synthesis of neurotransmitters that are important in memory processing [15]. Therefore, physical activity probably increases memory and cognitive function via different mechanisms.
Since humans cannot spend much time during the day for exercise [7]; in the current study, we used treadmill running because it is more similar to human exercise training, and allowed animals to run only for a limited time per day. Also, although exercise effects have been extensively studied; its effects on different periods of memory and together with with stress have remained largely unexplored. It is important to note that chronic stress is an unavoidable condition in society and a negative modulator of learning and memory process also accelerates the onset and severity of various brain disorders such as Alzheimer's disease (cognitive dysfunction)[16–18]. On the other hand the maintenance of brain health throughout life is an important aspect of public health. Since some studies indicated that exercise had beneficial effects on learning and memory, we investigated whether accompanying exercise with stress can improve memory deficit induced by stress? And can exercise protect the brain against destructive stress effects from the beginning? Or does exercise play the role of a stressor in stressed conditions. So the goal of the present study was to determine effects of synchronized exercise with stress on chronic stress–induced memory deficit.
METHODS AND SUBJECTS
Experimental animals
Experiments were performed on 50 male Wistar rats, with an initial weight of 250–300 g that were obtained from jondishapour Institute, Ahwaz, Iran. All of the experimental protocols were approved by the Committee of Ethics of the Isfahan University of Medical Science (Isfahan, Iran), followed the “Principles of laboratory animal care” and carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC). Five rats were housed in a cage; under light-controlled condition (12-h light/dark; lights on 07:00–19:00 h) in a room with a temperature of 22±2°C. Food and water were available ad libitum, except during the stressing procedure. Total duration of experiment was 42 days and the passive avoidance learning test was performed after 21 days. All behavioral experiments (passive avoidance test) were carried out at 13:00-14:00 h. Rats were randomly divided into five groups (n =10 in each) as follows:Control group (Co); rats were transported to the laboratory room and handled the same as the experimental animals throughout the study period and had no special treatment.Sham group (Sh); rats were put on the treadmill without running during 1h/day for 21 days.Exercise group (Ex); rats ran during 1h/day for 21 days on the treadmill.Under stress group (St); rats were under restraint stress during 6h/day for 21 days.Synchronized exercise with stress group (St&Ex); both exercise and stress were induced with the same as above protocol for 21 days.
Experimental procedures
Stress paradigms: In the current study, rats were placed in Plexiglas cylindrical restrainers and fit tightly into them during 6h/day for 21 days in the chronic stress model. It was not possible for them to move or turn around [19]. Hence, restraint was a powerful stress in rats [20]. The stress procedure was carried out in the institutional animal facility throughout the experimental period between 8:00 and 14:00.
Exercise paradigms: All rats in the exercised groups ran on a motorized rodent treadmill (Danesh Yakhteh Co., Tabriz, Iran). Adaptation to treadmill running was performed from three days before the experiments. In other words, three days before training, animals were left on the treadmill for 60 min once a day; while the treadmill was turned off. Then on the two remaining days before training, the treadmill was switched on and the speed increased from 5 to 20 m/min and the duration increased from 15 to 30 min. The exercise protocol consisted of 1 h/day/6 consecutive days at 20-21 m/min [21, 22], 0° slope, for 21 days running. Rats ran on the treadmill between 07:00 and 08:00 h. They were forced to run at the speed of the treadmill and received a mild electric shock (about 0.3 mA) from the grid, located just behind the treadmill. Electric shocks were used sparingly to motivate the animals to run [6]. at the beginning of the experiment onwards, after warm-up, speed and duration were kept constant at 20 m/min and 60 min per run. The stress associated with the likelihood of getting shocked was controlled by exposing the Sham groups to the treadmill apparatus without switching on the treadmill. These rats would receive the same electric shock when they stepped onto the grid.
Behavioral apparatus and method
Memory function was evaluated by the passive avoidance (PA) test at the end of experimental periods. This test, based on negative reinforcement, was used to examine the memory [23] and had been widely used to evaluate rodent memory ability in association with cortical and hippocampal functions [24]. The passive avoidance apparatus (Shuttle box 75×20×15cm) was divided into two compartments that had a grid floor and wooden walls. It consisted of a small light compartment (25×25×20 cm) and a larger dark compartment (50×25×20 cm). The two compartments were separated by a sliding guillotine door. On the first day, each rat was placed in the apparatus without electric shock for 5 min to habituate to the apparatus. On the second day, an acquisition trial was performed; rats were placed individually in the light compartment for 1 min and then the guillotine door was raised, when the rat entered the dark compartment, the door was closed and an inescapable scrambled single foot electric shock (50 Hz, 0.2 mA, 3s) was delivered through the grid floor by an isolated stimulator. In probe trials, the rat was placed in the light compartment again with access to the dark compartment without any shock. The delay of entering the dark compartment from light compartment was recorded as latency (up to a maximum of 300s); each rat had three trial sessions on 1, 7 and 21 days after the acquisition trial test for evaluating short, mid [25] and long-term memory, respectively. If an animal did not enter the dark compartment within 300s, the trial was terminated [26]. Absence of entry to the dark compartment or a longer duration in the light compartment indicated a positive response [4]. The PA task determines the ability of a rat to remember a foot shock delivered.
Data analysis
Latency of entrance to the dark compartment of the passive avoidance test (within groups) was analyzed using a Kruskal-Wallis nonparametric one-way analysis of variance, followed by a two-tailed Mann-Whitney U test. The comparisons of retention time 1, 7 and 21 days after foot shock (within groups) were analyzed by the Friedman test, followed by a Wilcoxon signed ranks test. All data are reported as the mean± SEM. A P-value less than 0.05 (P<0.05) was considered as significant.
RESULTS
The latency (delay time to entrance in dark compartment) in different time intervals (1, 7 and 21 days after foot shock) was measured in all groups.
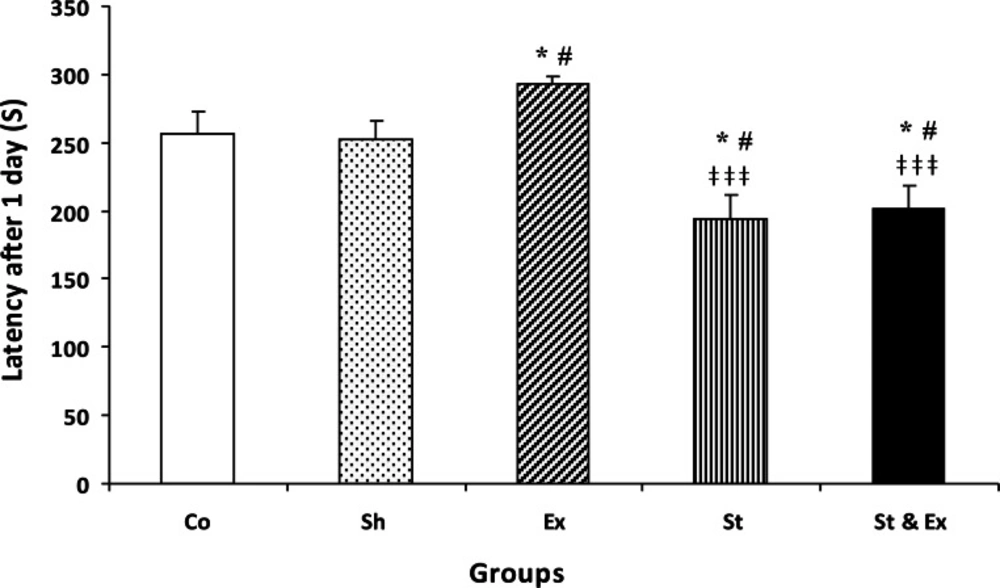
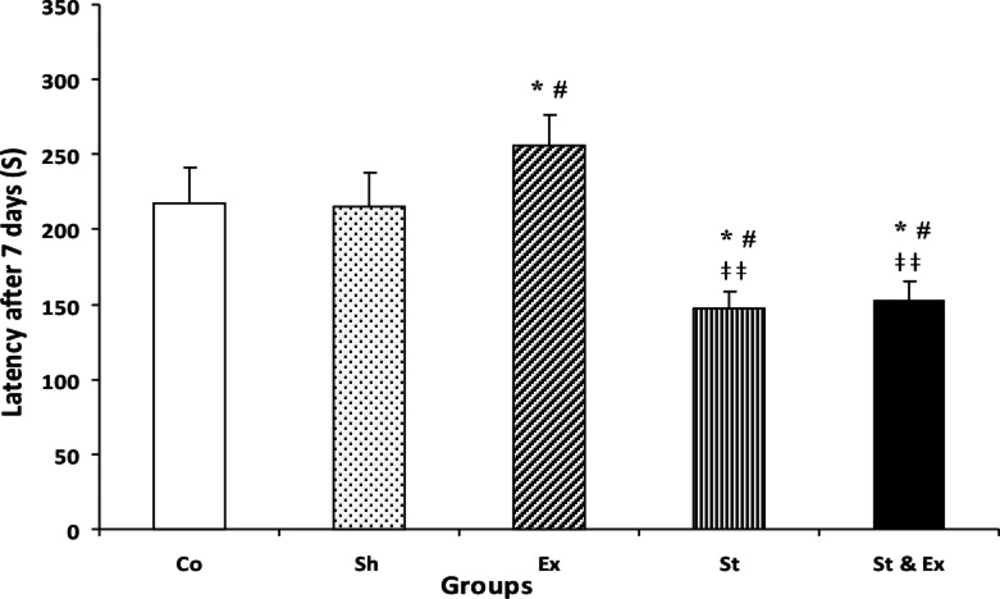
Results indicated that there were not significant differences between Control (Co) and Sham (Sh) groups in the latency of each of the three trials, indicating that the treadmill electric shock had no significant effect on memory deficit. The latency of the Exercise (Ex) group was significantly (P<0.05) higher than Co group in both trials 1 and 7 days (Figs. 1 and 2). The latency of Stress (St) group was significantly (P<0.05) lower than Co group in both trials 1 and 7 days (Figs. 1 and 2). The latency of the Synchronized exercise with stress (St & Ex) group significantly (P<0.05) differed from Co group in trials of the 1st and 7th day (Figs. 1 and 2).
Latency of entrance to the dark compartment of passive avoidance apparatus during memory retention test 1 day after receiving foot shock. Data represent mean ± SEM (n=10). Co/Sh groups vs. Ex group, in both them *P<0.05, #P<0.05, respectively; Co/Sh groups vs. St group, in both them *P<0.05, #P<0.05, respectively; Co/Sh groups vs. St&Ex groups, in both them *P<0.05, #P<0.05,respectively; Ex group vs. St and St&Ex groups, in both them ‡‡‡P<0.001; St group vs. St & Ex group, P>0.05 and nonsignificant; Kruskal -Wallis test, Mann-Whitney U test.
Latency of entrance to the dark compartment of passive avoidance apparatus during memory retention test 7 days after receiving foot shock. Data represent mean ± SEM (n=10). Co/Sh groups vs. Ex group, in both them *P<0.05, #P<0.05, respectively; Co/Sh groups vs. St group, in both them *P<0.05, #P<0.05,respectively; Co/Sh groups vs. St&Ex groups, in both them *P<0.05, #P<0.05, respectively; Ex group vs. St and St & Ex groups, in both them ‡‡P<0.01; St group vs. St & Ex group, P>0.05 and nonsignificant; Kruskal -Wallis test, Mann-Whitney U test.
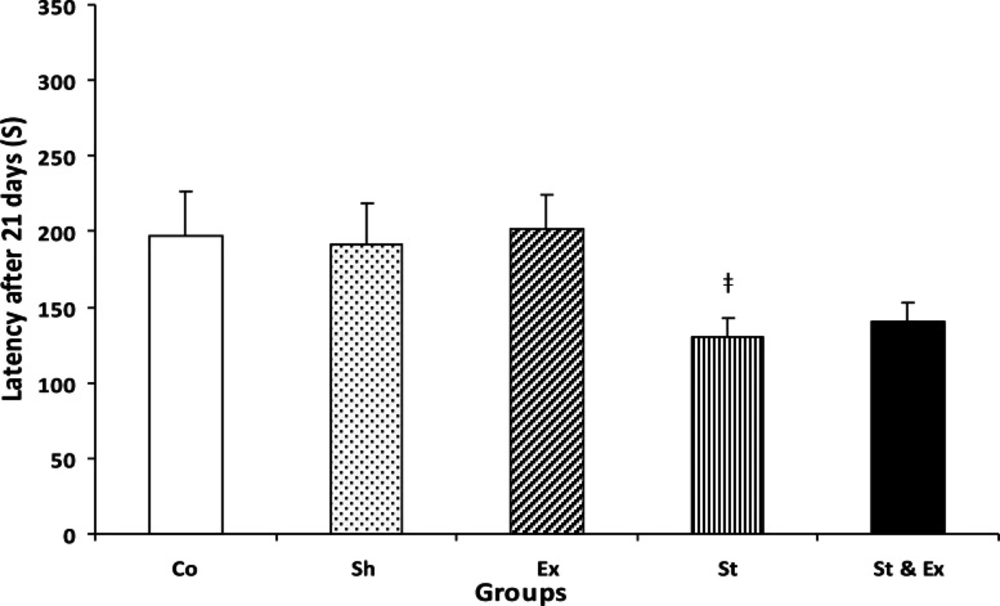
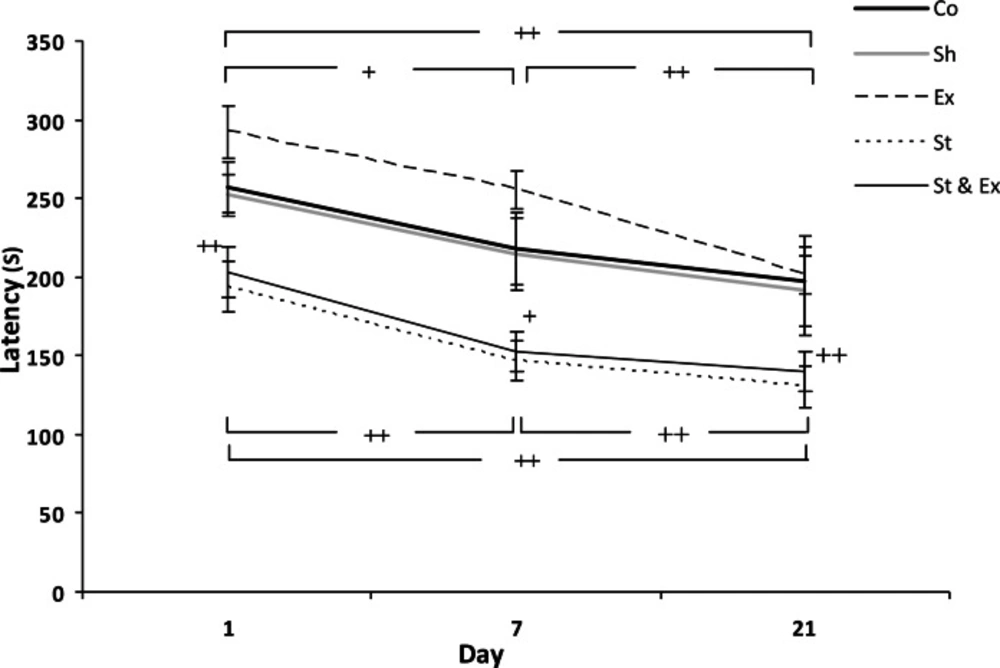
The latency of St and St & Ex groups had not significantly decreased during the tertiary trial (21 days after foot shock) when compared with Co group, it Co: Control group; Sh: Sham group; Ex: Exercise group; St: Stress group; St&Ex: Synchronized exercise with stress group. indicated that time passage caused memory decline (Fig. 3). The latency of Ex group had significant (P<0.001 and P<0.01, respectively) increase during the first and second trials (1 and 7 days after foot shock) when compared with St and St & Ex groups; Figs. 1 and 2). The latency of Ex group was significantly (P<0.05) higher than St group in 21 days after foot shock (Fig. 3). The latency of all trials in St & Ex group did not show significant increases when compared to St group (Figs. 1, 2 and 3). Latencies each three trials were *P<0.05 with respect to the Control group;#P<0.05 with respect to the Sham group;ǂ ǂǂP<0.01 with respect to the Exercise group analyzed by related sample to evaluate within groups latency changes; hence in this study, latency of 1 vs 7 days, 7 vs 21 days and 1 vs 21 days were compared (Fig. 4).
Latency of entrance to the dark compartment of passive avoidance apparatus during memory retention test 21 days after receiving foot shock. Data represent mean ± SEM (n=10). Co/Sh groups vs. Ex, St and St&Ex groups, in all of them p>0.05 and nonsignificant; Ex group vs. St group, ‡P<0.05; St group vs. St & Ex groups, P>0.05 and nonsignificant; Kruskal -Wallis test, Mann-Whitney U test.
Latency of entrance to the dark compartment of passive avoidance apparatus during memory retention test 1, 7 and 21 days after receiving foot shock (within groups). Data represent mean ± SEM (n=10). In Co/sh groups, latency of 1 vs. 7 days, latency of 7 vs. 21 days, latency of 1 vs. 21 days, P>0.05 and nonsignificant; In Ex group, the same of comparisons, +P<0.05, + +P<0.01, + +P<0.01,respectively; In St group, All of comparisons, + +P<0.01; In St& Ex group, the same of comparisons, + +P<0.01, +P<0.05, + +P<0.01,respectively; Friedman test, Wilcoxon signed ranks test
In this section, our data showed that there were not significant differences in latencies 1 vs 7 days, 7 vs 21 and 1 vs 21 days after receiving electrical foot shock in Co and Sh groups (Fig. 4). Latency of paired trials 1 vs 7 days in Ex and St & Ex groups showed significant (P<0.05 and P<0.01, respectively) decreases (Fig. 4).
In these groups, when the latency of 7 vs 21 days were compared; they had significant (P<0.01 and P<0.05, respectively) decreases (Fig. 4). Also, in Ex and St & Ex groups, latency of 1 vs 21 days showed significant (P<0.01) decreases in both (Fig. 4).
In St group when the latency of all paired trials (1 vs 7 days, 7 vs 21 days and 1 vs 21 days) were compared; they showed significant (P<0.01) decreases (Fig. 4). In general, memory had a descent trend with time passage in all groups (Fig. 4). In Ex group, descent trend in detraining period was more serious (Fig. 4).
DISCUSSION
The present results demonstrated that running activity had beneficial effects on memory, especially on short and mid-term (Figs. 1, 2 and 3). On the other hand, unexpectedly, it's helpful effect observed in detraining period. Several studies showed positive effects of physical activity that were in agreement with our results [27–31]. Mechanisms of exercise effects on brain function are different. It may result from structural and biological changes in the brain [32] including enhancing the neuron number [33], an increase in cell proliferation or decrease in cell death [34]. Exercise also increases the length and number of dendrite connections between neurons as well as synaptic plasticity in different regions of CNS such as hippocampus that is involved in memory [35–37].
The present study also showed that the restraint stress caused a significant decrease in latency of entrance to the dark compartment, which demonstrated severe memory impairment in rats (Fig. 4). Indeed, chronic stress is a negative modulator of learning and memory process [17, 38] which accelerates the onset and severity of cognitive dysfunctions [39]. Similarly, Rao et al indicated that chronic restraint stress impairs spatial learning and memory in rats [40]. Several researches on rodents and humans also have demonstrated that stress is a significant factor that can alter brain cell properties, learning and memory [41, 42]; however, stress can make different responses in different persons with the same stimulus and rate [43]. In our results, restraint stress had more serious effects on short and mid-term avoidance memory deficit (Figs. 2 and 3).
Since stress has great prevalence in society and can cause different neuropsychological disease, the maintenance of brain health throughout life is an important public aim; physical activity can probably help. So, in this research, the relationship between synchronized forced exercise with stress on chronic stress–induced memory deficit was investigated. These are novel findings, as we have found no similar reports in the literature.
Our results unexpectedly revealed that latency of entrance to the dark compartment was slightly enhanced by running activity in stressed rats in all probe trials (1, 7 and 21 days after foot shock). In other word, synchronized forced exercise with stress (St&Ex group) had not significant differences during all probe trials when compared with stressed rats (St group), also couldn't compensate memory deficit in stressed rats when compared with control group (Fig. 4). It indicated that synchronized forced exercise did not have the protective effects on stress- induced memory impairment (Figs. 1, 2 and 3) and could not improve cognitive function. Collectively, these results showed that effects of stress are more serious than exercise on memory in all trials (Figs. 1, 2 and 3).
There are probably several assumptions: I) Chronic stress has a much greater effect than exercise on memory that can overcome exercise beneficial effects. II) Simultaneous exercise probably makes biochemical changes in different regions of the brain as to structural changes such as synaptic plasticity, enhancing the neuron number [33], increases of spine numbers [35–37] induced by exercise [32]. III) Stressed animals have threshold for endurance of stress, and so synchronized exercise with stress causes an increase in stressor condition, although it showed a little increase. Therefore, the dual effects restraint stress and exercise may create conflicting physiological changes that could adversely affect physical activity outcome. Hence, latency changes may be related to release of multiple transmitters in some region of brain that related to memory. Thus the balance between beneficial and potentially harmful effects of exercise might specially be of importance during a stress condition. It is possible that the mechanism of simultaneous exercise with stress effects may be due to interaction of some neurotransmitters with each other and/or enhancing oxidant status of the body. Fontella and et al reported repeated restraint stress induces oxidative stress in the rat hippocampus [44]. Several other studies suggest that exhaustive exercise may promote free radical production and oxidative stress [45]. Therefore, synchronized exercise with stress probably can be exhaustive and increase range of oxidative stress in brain in a way that it could not improve stress effects. There is a possibility that the complex interaction between brain neurotransmitters and their specific receptors could play a role in the onset of fatigue during prolonged and synchronized exercise with stress [22]. In contrast to our results, some researches address the positive effects of exercise on emotional and cognitive functions [46–48] but this was not in agreement with simultaneous exercise with stress in this study (Figs. 1, 2 and 3).
According to the above results, synchronized running did not have significant positive effects on encountering memory impairment in stressed rats in long duration and probably can only prevent from more serious damage of brain induced by chronic stress. In this way, some studies indicated that running activity can protect neurons from various forms of brain damage and has helpful effects on neural health and function [49, 50].
Our finding proposes that exercise duration with respect to stress period is considerable. Hence, it may offer that the influence of exercise in stressful conditions was probably time-dependent on memory estimated by passive avoidance performance (Fig. 4). Whereas some studies have consistently demonstrated that exercise has the beneficial effects on different aspects of cognition, differences may depend on factors such as time of doing exercise, the duration of exercise exposure, type of exercise undertaken (e.g. forced vs. voluntary), task difficulty or other variables that have not yet been defined [51]. Our results showed that memory had descending trend with time passage in all groups, although they were not significant in Co and Sh groups (Fig. 4).
Previous studies reported that exercise improved memory in normal and some disorders in rats [27, 28, 52], but all of them did not use synchronized exercise.
CONCLUSION
Our results at the behavioral level emphasize that aerobic exercise doesn't have significant positive and protective effects on memory deficit in stressed rats but it has a better effect than no training on memory deficit in stressed rats. Therefore it can only prevent from more serious stress-induced memory deficit. The present study supports the hypothesis that the synchronized exercise may reflect different responses on memory deficit in stressed rats, and suggests a strong relationship exists between stress and exercise. Therefore it seems that synchronized exercise with stress is not the complete exercise program and may create conflicting physiological demands. Thus the balance between beneficial and potentially harmful effects of exercise might be important in stress conditions. It proposes that further research needs to determine memory mechanisms such as signaling system in the different phases of memory in synchronized exercise and also progressive exercise must be investigated.