INTRODUCTION
Sarcoidosis is a multisystem disease with unknown etiology, characterized by the presence of “noncaseating granulomas” in several organs. Despite the fact that exact prevalence of sarcoidosis is not known yet, its incidence in people like black Americans and Scandinavians is higher than others[1, 3]. The greatest incidence has been observed in young adults aged between 20 and 40 years. Athletes can also be involved with an evident reduction in their sport performance. For almost 50% of the patients the diagnosis is often casual or occasional in case of the evident pulmonary radiographic abnormalities. Actually, the accurate etiology of the disease is not exactly known. It has been supposed to be mainly found in susceptible subjects as a consequence of an inflammatory response of organism to external inputs. The possible causes of immunologic response could be infections (viral, bacterial or mycobacterial) or inhalation of organic and inorganic agents. The inflammatory response is therefore characterized by accumulation of T-lymphocytes and macrophages with consequent organization of granulomas[4]. According to the current guidelines[4], diagnosis of Sarcoidosis is actually based on the presence of typical symptoms (fever, fatigue, malaise, asthenia, dyspnea, cough, polyarthralgia, lymphadenopathy), high plasma level of a peculiar enzyme i.e. Angiotensin Converting Enzyme (ACE), presence of a bilateral hilar lymphadenopathy at the standard chest radiological exam and histological evidence of noncaseating epithelioid cell granulomas[4]. In order to confirm the diagnosis, some additional laboratory and instrumental exams (Purified Protein Derivative (PPD) skin test; serum level of erythrocyte sedimentation rate (ESR), C-reactive protein (PCR), Calcium, Phosphate; Kveim reaction test; complete microbiologic tests, Holter monitoring, echocardiography and thallium or gallium scans, magnetic resonance imaging (MRI), CT scans of the central nervous system and myocardial involvement can be helpful[4]. In spite of the fact that the most typical symptoms of sarcoidosis include cough, dyspnea, thoracic pain, fever and the weakness, a substantial absence of symptoms can be found in 90% of the patients, where bilateral hilar adenopathy and interstitial or alveolar opacities are often evident. Just in cases with big conglomerate clusters of granulomas, especially in the upper parts of lungs, due to the presence of the stenosis or bronchiectasis, the symptoms can be found[5]. Otherwise, involvement of different organs and tissues in athletes is frequent: in 10% of them the skeletal-muscles show acute polyarthritis, especially in hips. Besides, tendons and peritendinous tissue can be affected by a degenerative process that can result in breakage, especially in cases of protracted stress. The cardiac injury occurs more frequently in Japanese female population (approximately 20-47% of patients). This aspect determines 85% of deaths from sarcoidosis. However, the symptoms are observed only in 5% of cases and the survival up to five years is evident in about 60% of the subjects[6]. A cardiac sarcoidosis characterized by wide fibrotic areas, can involve the pericardium, the endocardium, or the myocardium areas, mainly in the free wall of the left ventricle and in the interventricular septum creating some possible arrhythmias (from a first grade atrioventricular block to a complete block, atrial arrhythmias, sustained or non-sustained ventricular tachycardia) due to a reentry loop[7]. A potential evolution toward the left ventricle dilatation with heart failure has also been described (8,9,10). Diagnosis of cardiac sarcoidosis is possible by the electrocardiographic, echocardiographic and radionuclide investigations (Gadolinium MRI exam), while the gallium-67 citrate (Ga) or Thallium-201 scintigraphy are normally used to assess the activity of the disease and the response to the corticosteroid therapy[11, 13]. Cardiac biopsy is normally avoided because of the high level of risk associated with a low sensitivity[14]. In conclusion, the chest XR is generally the first exam to support the diagnosis of sarcoidosis. However, in case of doubt, a chest CT in addition to the bioptic sampling can be helpful to identify the granulomas and also to perform the differential diagnosis among other “granulomatous diseases”. The respiratory functional tests remain however the main tests to estimate the disease's severity, showing normal values at the first stages and a restriction pattern with a reduced diffusion of CO in advanced stages[15].
CASE PRESENTATION
The patient gave oral consent to participate in this case report. Since October 2008 a 33-years-old Scandinavian professional soccer player has shown splitting headache with fever and meningitic irritation signs like photophobia.
The first diagnostic hypothesis was addressed toward a Toscana Virus infection that occurs frequently in this area. Initially, all serologic tests were negative. Head CT scans and magnetic resonance angiography (MRA) were negative either. Only a slight increase of ACE and unspecific flogosis-indexes like ESR and CRP were evident. Despite these results, he was submitted to a corticosteroid therapy with the resolution of the headache and fever. However, considering the persisting strong weakness with an inevitable negative impact on his sport performance, some other possible causes for the asthenia were considered and evaluated following an additional diagnostic flow-chart. The examinations made during one more month spent in the hospital showed normal values of the general blood tests, no serological signs of lymphoma along with a little increase of ACE plasma level. The serologic tests by polymerase chain reaction technique (PCR) including PPD skin test resulted to be negative except a past Toscana Virus (IgG) infection.
Among the cardiological investigations, the echocardiographic exam was normal while Maximal Ergometric Test (MET) showed sporadic singular monomorphic ventricular extrasystoles. In addition, 24-hour Holter-monitoring ECG showed some polymorphic ventricular extrasystoles, partially organized in short bigeminal rhythm, and monomorphic and polymorphic couples at rest condition.
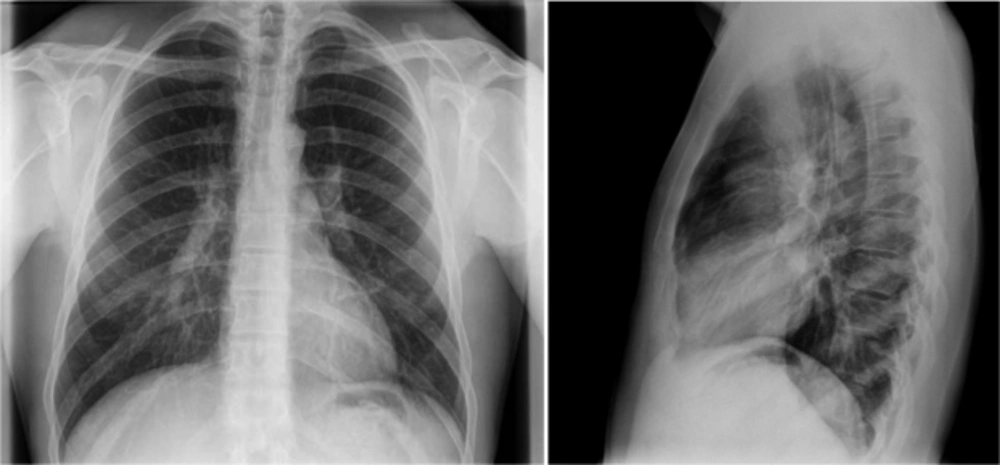
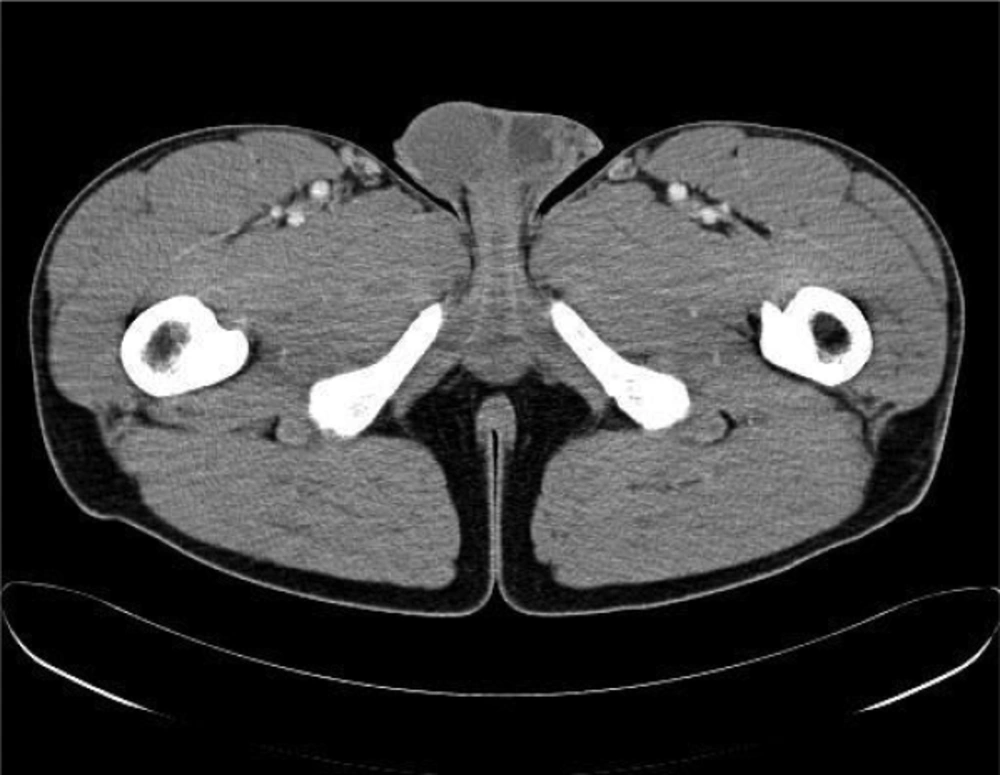
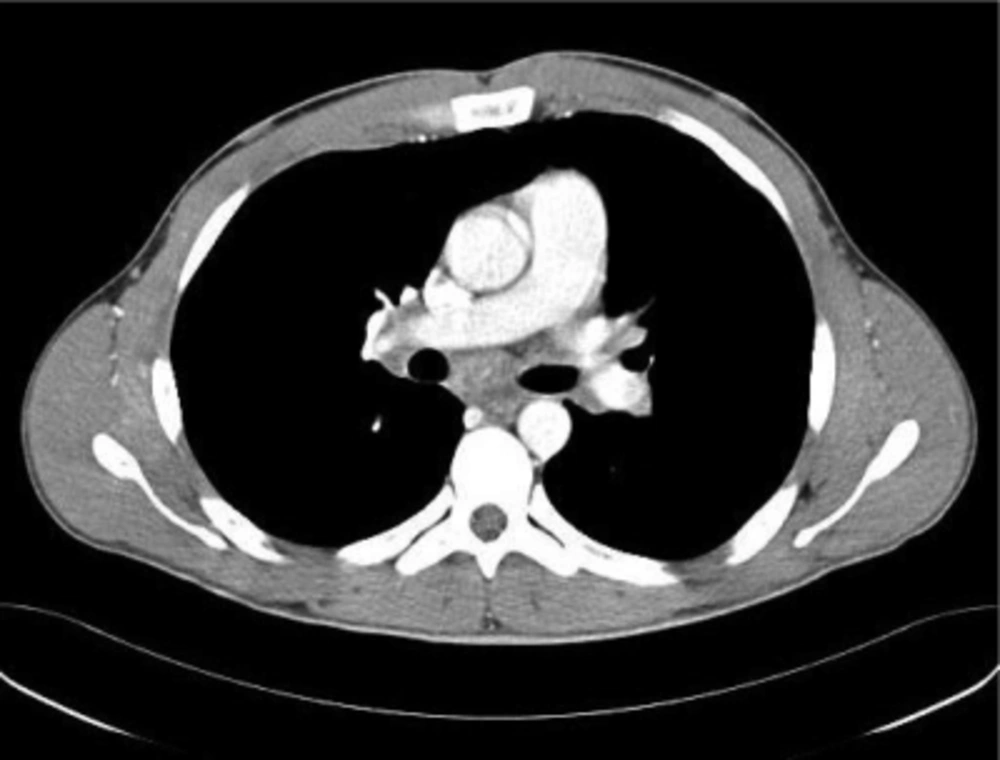
The respiratory functional tests described a pattern compatible with a slight obstruction and a normal CO diffusion. The chest XR was characterized by a moderate parenchymal involvement due to a hilar lymphadenopathy (Fig. 1). The abdominal echography showed an enhancement of the liver and spleen dimensions at the presence of abdominal lymph nodes, presence of which was confirmed by the abdominal and chest CT (Fig. 2). Increase of inguinal, mediastinal, supraclavicular and cervical lymph nodes with the same pattern were also described (Fig. 3). The additional evaluation by Positron Emission Tomography (PET/CT) showed a mediastinal and supraclavicular areas with higher glucose-metabolism than others. In the meantime, the athlete was quite asymptomatic and the weakness was the sign more constant. Therefore, diagnosis of sarcoidosis was supposed. The athlete was submitted to a bioptic sampling of the laterocervical lymph nodes confirming the diagnosis previously hypothesized. Biopsy showed “noncaseating granulomas”.
Considering the quite good clinical condition of the athlete, no more additional pharmacologic therapy except one month of detraining was advised. He was in a fairly good physical state thirty days later, and the follow-up exams didn't show any more progression of the disease. He gradually restarted his training after four months and he actually was in good performance.
DISCUSSION
The symptom “asthenia” in a professional athlete is often described as loss of his global performance. In case of “unspecific symptoms” like headache, muscular fatigue and fever, it is necessary to reduce the time of sportive seasonal training to avoid the negative consequences of the overtraining. When it is possible to exclude any relation between symptoms and sport activity, it is correct to suspect a common pathology as flu syndrome or infective pathologies. Otherwise, according to previous examples of cases reported[22, 23], where exclusively unspecific clinical symptoms are evident and the most common pathologies can be excluded, some other diseases as lymphoma, tuberculosis, rheumatoid arthritis, rheumatic fever and mycosis have to be considered. Besides, when these ones can be excluded, it is consequently reasonable to think of sarcoidosis. Fatigue is the most common and the major problem in young patients with sarcoidosis, submitted or not submitted to a corticosteroid therapy. As no direct relation is often evident between fatigue and clinical parameters, it is very difficult to evaluate this aspect. No standard quantification of these symptoms has been determined yet. The Fatigue Assessment Scale (FAS), and the 6-minute walk[15, 17] can be considered now as the exclusive valid instruments to assess this one and are able to show the impaired exercise tolerance due to multiple factors above[18, 20]. Sarcoidosis can often involve unexpected organs[22, 23] and only the evidence of noncaseating granulomas can confirm the doubt.
Correct diagnosis of sarcoidosis is therefore possible following a flow-chart to obtain a complete differential diagnosis panel, considering the presence of symptoms, the radiologic signs, in addition to a histopathologic confirmation. After the anamnesis and the physical examination several other exams are necessary:- Complete blood tests- ECG, echocardiogram, ECG sec. Holter- Respiratory functional tests- Chest XR- Chest CT: especially in advanced stages of disease, when the radiologic images can simulate pulmonary metastasis- MRI or scintigraphy with Gallium-67 or Tallium-201- Transbronchial bronchoscopy and biopsy
Ninety percent of patients have a spontaneous resolution after two years, while restarting the disease is evident in less than 10% of them. On the contrary, sarcoidosis becomes chronic with permanent issues in 30% of the cases. The symptomatic subjects are normally submitted to a corticosteroid therapy (Prednisone 0.3-1 mg/Kg/day until a maximum of 40 mg/day). If the patient shows a good resolution of the disease within the first two weeks of therapy, he can be considered a “responder”. When the corticosteroid therapy is interrupted, restart of the disease activity is possible.
In cases of side-effects or if the athlete is “non-responder”, an alternative therapy can be tried with azathioprine, cyclophosphamide, chlorambucil, chloroquine, pentoxifylline or infliximab[21, 14]. The rate of the follow-up depends on stage and degree of the disease: every 4-8 weeks in subjects with acute sarcoidosis and corticosteroid therapy can be sufficient, whereas a 3-4-month period is acceptable in the first years and more rarely afterwards if the disease is indolent[21].
CONCLUSION
The data obtained show a “sarcoidosis on first stage”, which has not been developed yet and is exclusively characterized by clinical manifestations, i.e. the persistent asthenia.
This case report supports the assumption that therapy is not normally necessary in asymptomatic subjects. Respiratory functional tests and blood markers of disease are often sufficient, especially when the symptoms are restricted to a weakness. A different period of detraining is, in fact, sufficient to restitute the performance previously lost.