1. Background
The relationship between parasympathetic (PNS) and sympathetic nervous system (SNS) activation can be assessed with heart rate variability (HRV) analysis (1, 2). Increasing the exercise intensity may thus reflect a reduction in HRV during the initial stages of a maximal incremental exercise test, thereafter a significant PNS activation withdrawal occurs between 50% - 60% of the maximal oxygen uptake (VO2MAX) (3). That intensity has been related with the first physiological threshold (i.e. first lactate or ventilatory threshold - LT1 and VT1, respectively) (4). In contrast, there is a gradual increment in SNS activation from intensities corresponding to LT1 and VT1, with remarkable increase at exercise intensities related to the second physiological threshold (i.e. second lactate and ventilatory threshold - LT2 and VT2, respectively) (3, 5). As a result, studies have suggested that HRV analysis may be a potential and useful tool to estimate the exercise intensity of the first and second physiological thresholds (4, 6-8).
Using a cycling exercise mode, Cottin et al. (7) found that nonlinear increases in HRV data, assessed by high frequency (HF) and peak frequency spectrum (ƒHF) of the beat-to-beat RR intervals, identified VT1 and VT2. They also showed that instantaneous HF multiplied by ƒHF (HF.ƒHF) provided a reliable and accurate index to identify these thresholds. In a subsequent study, these authors expanded these findings to running exercise mode. Using an exhaustive incremental running test performed on a 200 m track, Cottin et al. (8) showed that VT1 and VT2 could be assessed through a simple HRV monitor. Recently, these results were further confirmed in obese individuals (9), corroborating the usefulness of the HRV method to identify the first and second physiological threshold in different individuals, during different exercise modes.
However, despite the support to use HRV data set when determining VT1 and VT2 (7-9), some aspects should be addressed. No study has currently verified the agreement between breakpoints identified through HRV and LT1 and LT2, as no lactate measures were included in those previous studies (7, 8). In this regard, it should be highlighted that lactate thresholds (10-13) is considered as a gold standard method when predicting running aerobic performance. The agreement between ventilatory and lactate thresholds is still an ongoing debate, as these indices are used to identify identical exercise intensities. Since work by Gaesser and Poole (14) nearly thirty years ago, studies have shown different results when comparing VT1 and VT2 to LT1 and LT2, respectively (15-17).
On the other hand, Karapetian et al. (4) have suggested the identification of a HRV threshold by using time domain indexes (standard deviation - SD and mean successive difference – MSD), which may match lactate and ventilatory thresholds during maximal incremental cycling test. Furthermore, it is important to point out that those studies have reported a nonlinear pattern in HRV data during maximal incremental exercise tests, thus making possible the identification of a HRV threshold when visually inspecting abrupt breakpoints in HRV responses during maximal incremental exercises. Consequently, it has been suggested that the HRV threshold, regardless of the method used for its determination, may be used to estimate the first and second physiological thresholds with different methodologies of HRV data analysis such as frequency domain (7, 8), time domain (4) or Poincare plot (18). Unfortunately, however, an important aspect that remains to be confirmed is the possibility of estimating LT1 and LT2 by using HRV data set in incremental running test.
The use of HRV data set for estimation of these traditional thresholds may be of interest, as this method is less expensive and time consuming, and requires uncomplicated and accessible HR monitors. Traditional VT1 and VT2 determination requires sophisticated and expensive metabolic gaseous exchange analyzers, while the LT1 and LT2 require invasive procedures with multiple blood sample collections, making these methods impractical for clinical assessment and daily endurance training sessions. Therefore, the study of alternative HRV methods for estimation of the first and second physiological threshold has practical relevance.
Reliability of the HRV thresholds method has been provided during maximal incremental cycling tests (18), but no study has been designed yet, to verify the HRV thresholds’ validity and reproducibility when determining LT1 and LT2 in maximal incremental running test. Thus, the purpose of this study was to determine if HRV thresholds could identify the lactate thresholds’ intensity during a maximal, incremental running test. We expected that HRV thresholds may elicit reliability and reproducibility when identifying lactate thresholds in treadmill running test.
2. Objectives
Therefore, the first aim of this study was to verify if the HRV method would determine valid HRV breakpoints during running test and if these corresponds to the first and second physiological threshold such as LT1 and LT2. The second aim of this study was to verify the reproducibility of the HRV method in running test.
3. Methods
3.1. Participants
Nineteen recreational long-distance male runners (30.4 ± 4.1 years; body mass of 74.3 ± 8.5 kg; height of 176 ± 6.4 cm and body fat of 13.8 ± 4.6 %) volunteered to participate in this study. All participants were healthy, non-smokers, with no cardiovascular or neuromuscular diseases. They were informed about risks and benefits before participating in the study, thereafter they provided their written informed consent. The study protocol was approved by the institutional ethics committee of the University of São Paulo, and conducted according to international standards (19).
3.2. Study Design
The experimental design consisted of two maximal incremental running tests interspersed by a washout period of 3 - 7 days. The tests were performed at the same time of the day and in standard laboratory conditions (humidity of ≈ 50% and temperature of ≈ 22°C). Participants were asked to avoid intense exercises, alcohol and caffeine beverages 24 hours before each test, and to consume a light meal 3 hours before the tests. Before starting the experimental procedures in each test session day, we asked participants about the accomplishment of these recommendations so that only participants who had followed them were evaluated.
3.4. Maximal Incremental Running Test Protocol
Participants wore a cardio belt for beat-to-beat heart rate (HR) measures (s810 Polar®, Kempele, Finland). Thereafter, the participants rested for 20 min (10 min supine + 10 min sitting) for baseline measures of HR and lactate concentrations. Lactate concentrations [La] were determined from a 25 µL blood sample, drawn from the tip of the forefinger (YSI 1500, Yellow Springs, OH, USA). Then the participants moved to the treadmill (CEFISE TK35, Nova Odessa, Brazil) and warmed up for 3 min at 5 km.h-1 and 1% gradient. The test started at 5 km.h-1, with 1 km.h-1 increases every 3 min, until exhaustion (10). The HR data set were recorded continuously throughout the tests, while 25 µL of blood samples were collected during the last 30 s of every stage, while the participant was running.
3.5. Determination of Physiological Thresholds
LT1 and LT2: The speed corresponding to a 2 mmol.L-1 blood lactate concentration was used to determine LT1 (20), while the speed corresponding to 3.5 mmol.L-1 identified the LT2 (10).
Dmax: The lactate threshold was further determined according to the Dmax method (LTDmax) suggested by Cheng et al. (11), thereby providing individualized lactate threshold values. Briefly, LTDmax reflects the longest perpendicular distance between [La] values predicted by a 3º order polynomial function over actual [La] values and values derived from a linear regression calculated with the outermost lactate values (i.e. the first and last values of that curve).
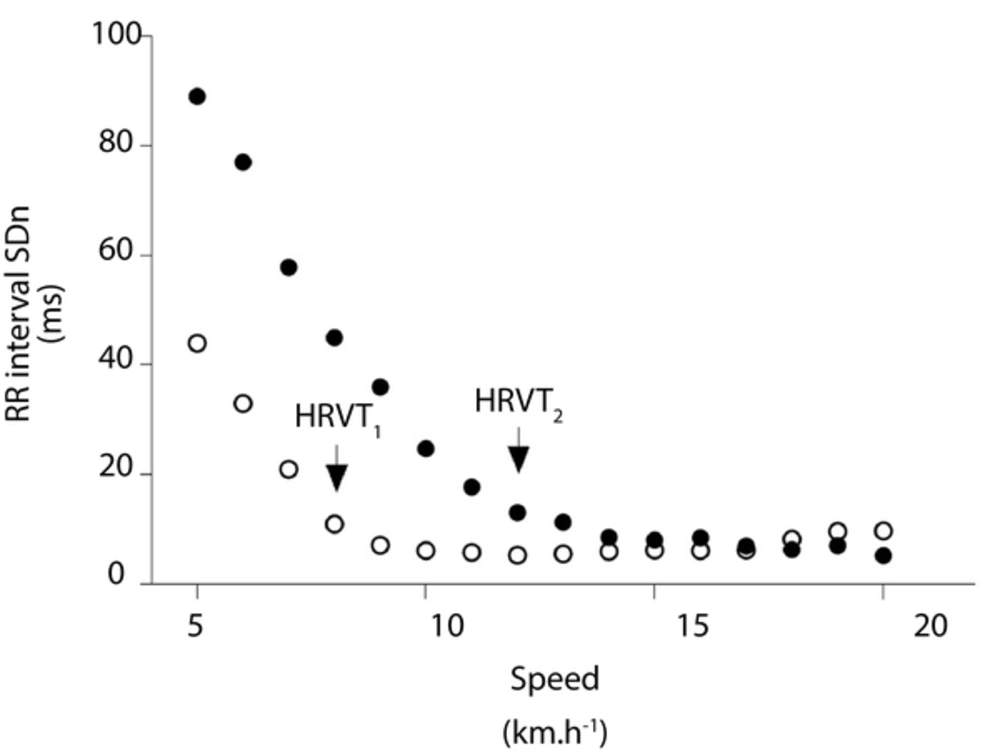
HRV thresholds: Beat-to-beat RR interval data were analyzed for aberrant values. A RR interval value was interpreted as a premature beat if it deviated from the previous interval by more than 30% (1, 4). The Poincare plot analysis was performed on RR interval time series to calculate the standard deviation of the instantaneous (SD1) and continuous long-term RR intervals (SD2) (3). Poincare plot is a nonlinear measure of temporal correlations within time series of inter-beat intervals in which an RR interval is plotted against its first predecessor. The SD1 has been shown to correlate strongly with vagal tone (PNS) and SD2 has been shown to correlate with the PNS and SNS. Thereafter, raw RR intervals recorded during the last 60 s of each stage of exercise were normalized by the mean RR interval and multiplied by 1000 (SD1n and SD2n) (1). Thus, in accordance with behavior of these variables in previous studies (3, 18), SD1n and SD2n were visually used by three evaluators to determine two HRV thresholds. The first (HRVT1) and second (HRVT2) HRV thresholds were identified at the first breakpoint in SD1n and SD2n curves, respectively. Figure 1 illustrates typical SD1n and SD2n curves and the HRV thresholds.
All physiological thresholds, LT1, LT2, LTDmax, HRVT1 and HRVT2 were expressed in absolute speed (km.h-1), HR (bpm), milliseconds beat-to-beat RR interval (ms) and blood lactate concentration (mmol.L-1). Additionally, thresholds were expressed relatively (%) to maximal values.
3.6. Statistics
Values were expressed as mean and standard deviation (± SD). After ensuring Gaussian data distribution (normality and homoscedasticity), a repeated-measure design ANOVA with Bonferroni’s post-hoc test were used to verify possible differences among different thresholds identified in maximal incremental running test. Typical error of measurement (21) was used to verify the test-retest HRV breakpoints’ reproducibility, as well as to compare them with lactate thresholds. The Bland and Altman plot further provided a visual analysis of the data set bias (22), in addition to the reliability analysis with intra-class coefficient correlation (ICC). All analyzes were performed with SPSS (19.0), and with a spreadsheet designed to specifically calculate the reliability (available on www.sportsci.org). Results were interpreted as significant when P was < 0.05.
4. Results
Baseline HRV values (423.1 ± 28 ms vs. 994.6 ± 139.3 ms; P < 0.05), but not baseline lactate values (1.34 ± 0.5 mmol.L-1 vs. 1.18 ± 0.3 mmol.L-1. P > 0.05), were significantly different between test and retest. With regard to values recorded at the exhaustion in maximal incremental running tests, no significant difference was observed between test and retest (P > 0.05) in speed (16.5 ± 1.8 km.h-1 vs. 16.6 ± 1.7 km.h-1), HR (192 ± 6 bpm vs. 191 ± 6 bpm) and blood lactate concentration (9.2 ± 1.9 mmol.L-1 vs. 9.2 ± 1.9 mmol.L-1).
4.1. Identification of Physiological Thresholds
Overall results were that neither the first nor the second. Physiological threshold was different when determined by lactate and HRV thresholds expressed in absolute as well as in relative values. For example, while LT1 matched HRV1, LT2 matched HRV2. Interestingly, LTDmax matched the first (LT1 and HRV1), but not the second physiological threshold (LT2 and HRV2.). Table 1 shows in full results of the first and second physiological thresholds identified during the maximal incremental running test.
| Speed | RR | HR | Lactate | |||||
|---|---|---|---|---|---|---|---|---|
| (km.h-1) | (%) | (ms) | (%) | (bpm) | (%) | (mmol.L-1) | (%) | |
| LT1 | 11.9 ± 3b,c | 71.4 ± 11b,d | 392.8 ± 48e | 92.9 ± 11e | 155 ± 17b,d | 80.8 ± 8b,d | 1.97 ± 0.33b,d | 30.3 ± 10.6b,d |
| LT2 | 14.2 ± 3 | 85.7 ± 7 | 348.4 ± 25 | 82.5 ± 5 | 173 ± 12 | 90.2 ± 5 | 3.20 ± 0.51 | 48.9 ± 15.4 |
| LTDmax | 12.3 ± 1 | 74.5 ± 4b,c | 376.5 ± 27 | 89 ± 4 | 161 ± 12b,c | 83.5 ± 5b,c | 2.25 ± 0.67b,d | 32.6 ± 7.2b,d |
| HRVT1 | 11.6 ± 2b,d | 70.6 ± 6b,d | 384.2 ± 38e | 90.8 ± 4e | 155 ± 15b,d | 80.7 ± 7b,d | 2.02 ± 0.68b,d | 29.3 ± 7.8b,d |
| HRVT2 | 14.2 ± 2 | 86.1 ± 6 | 349.6 ± 21 | 82.8 ± 4 | 173 ± 9 | 90.4 ± 5e,d | 3.42 ± 1.20 | 50.9 ± 18.3 |
aValues are expressed as mean ± SD.
bSignificant differences in relation to LT2 and HRVT2 methods.
cP < 0.05.
dP < 0.01.
eSignificant differences in relation to LTDmax methods. (Percentage values (%) are relative to baseline (RR) or maximal values (speed, HR and lactate).
Pearson correlation coefficient analysis in thresholds expressed as speed revealed significant correlation (P < 0.01) between HRVT1 and LT1 (r = 0.71), HRVT1 and LTDmax (r = 0.68), LT1 and LTDmax (r = 0.75), HRVT2 and LTDmax (r = 0.65). Additionally, significant correlation was observed between HRVT2 and LT2 (r = 0.86).
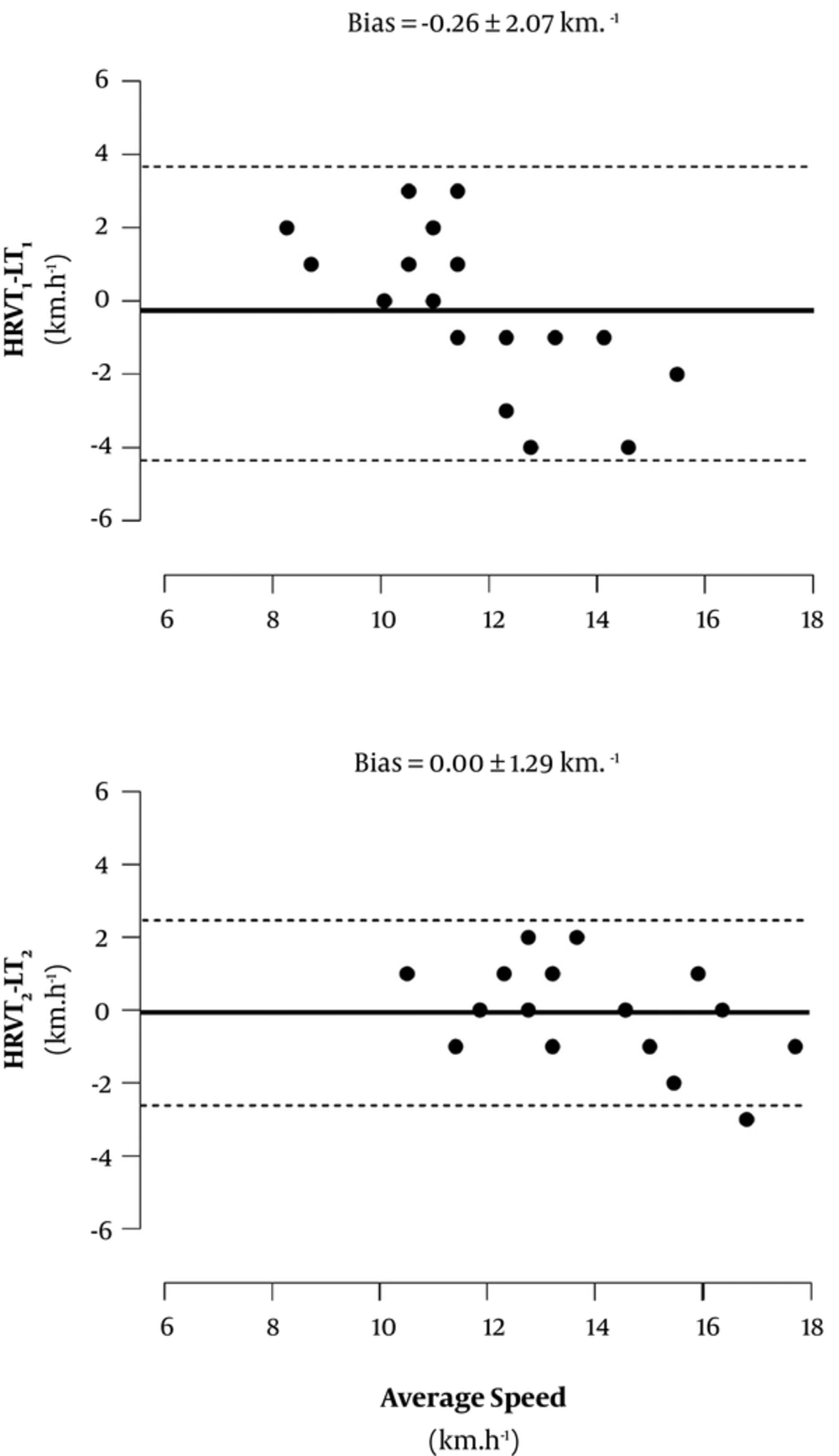
Figure 2 shows the magnitude of differences and bias between HRV and lactate thresholds. A good agreement was observed between HRVT1 and LT1 (-0.26 ± 2.07 km.h-1), as well as between HRVT2 and LT2 (0.00 ± 1.29 km.h-1), given the small bias reported.
4.2. Reproducibility of the HRV Thresholds
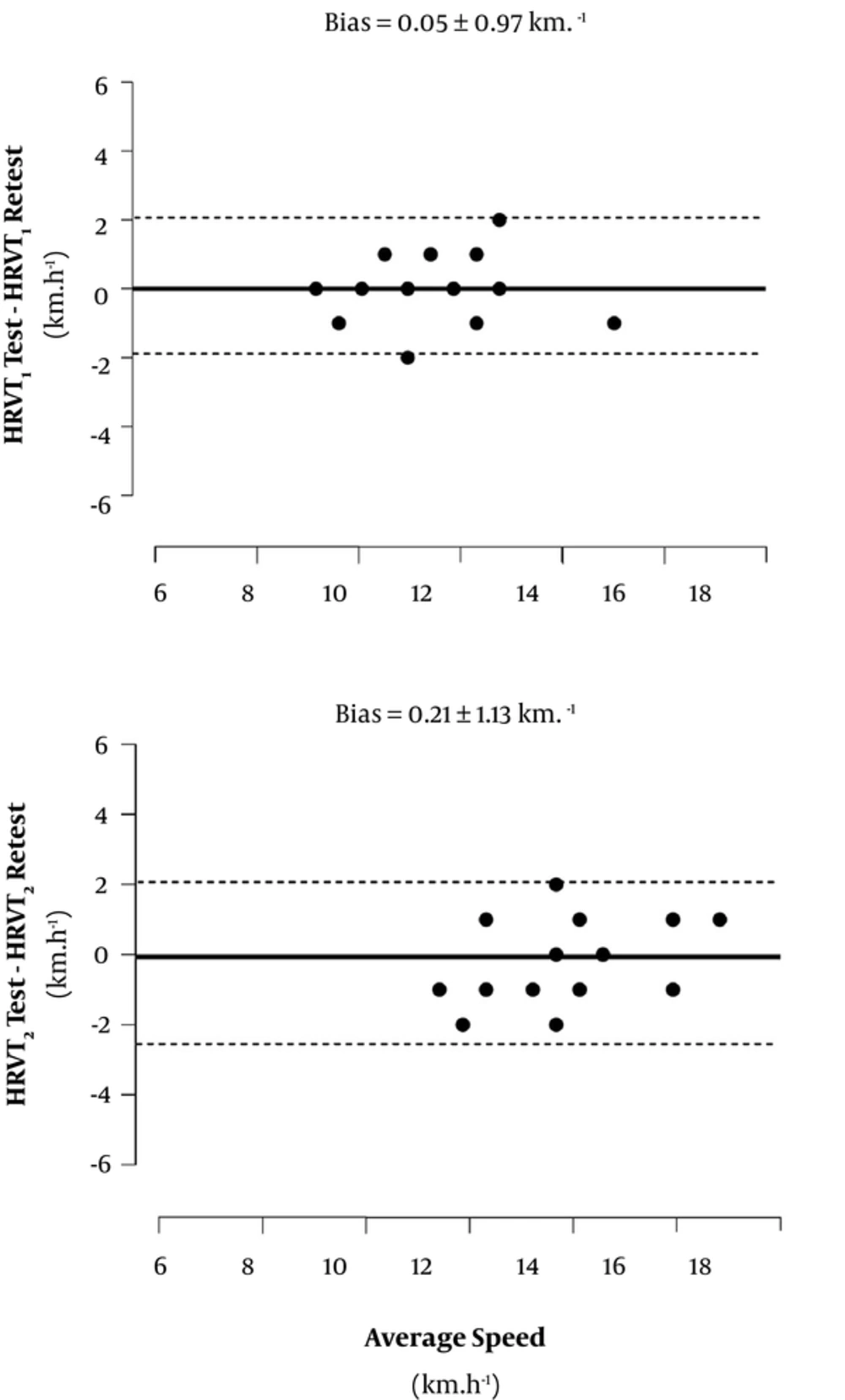
Figure 3 presents the Bland-Altman plot with bias analysis for the HRVT1 and HRVT2 reliability. There was a reasonable random distribution in test-retest residuals for HRVT1 and HRVT2, thus suggesting a homoscedastic distribution as a function of the speed. Results further suggested a consistent reproducibility for thresholds determined through the HRV data set, given the typical error of measurement reported at ≈ 6% (≈ 0.45 km.h-1) and the ICC showed high correlations (P < 0.001) (Table 2).
| Reliability Variables | HRVT1 | HRVT2 |
|---|---|---|
| TEM | ||
| Absolute, km.h-1 | 0.45 | 0.46 |
| Relative, % | 6.0 | 6.0 |
| ICC | ||
| Magnitude | 0.889 | 0.884 |
| P Value | 0.000 | 0.000 |
zAbbreviations: ICC, intra-class coefficient correlation; TEM, typical error of measurement.
5. Discussion
The main findings of the present study were that the HRV data set provided fair estimation of LT1 and LT2 through HRVT1 and HRVT2, respectively, during maximal running test. Furthermore, HRV thresholds elicited good reproducibility with low bias during incremental running test.
5.1. HRV Data Set and the Identification of Lactate Thresholds
The results of this study showed that HRVT1, identified by analysis on time domain of the HRV data matched LT1 and LTDmax, either expressed in absolute or relative values of speed and HR. The significant vagal withdrawal during incremental exercises has been well documented in the literature using time domain HRV analysis (4, 18, 23). In fact, HRVT1 showed relative values of speed around 70.6%, close to values (74.6%) reported by Candido et al. (18) when using a cycle ergometer and similar methods for HRV analysis. In the present study, although the correlation between either HRVT1 and LT1 or HRVT1 and LTDmax (expressed as km.h-1) has elicited significant coefficient values (r = 0.71 and r = 0.68, respectively), this was lower than the correlation reported by a previous study by Karapetian et al. (r = 0.82) (4) which used similar visual analysis to identify the vagal withdrawal, that is the point at which the HRV showed no further decrease. However, it is important to note that in contrast to the present study which used a treadmill running incremental test, Karapetian et al. (4) used a cycle ergometer, thus limiting deeper comparisons as the values were expressed as power output (watts) instead of speed (km.h-1).
Furthermore, others have shown that HRV is a useful method to identify the second physiological breakpoint (6-8). Accordingly, we showed that HRVT2 was similar to the second physiological threshold identified through the traditional lactate method (LT2). The variable SD2 was used because it represents the parasympathetic and sympathetic nervous system activity, and this variable shows a non-linear pattern in intensities close to heavy and severe domains (3). At intensities above the first physiological threshold as the LT1 the sympathetic system starts to intensify its activation (24), so that a significant activation occurs in intensities close to the second physiological threshold, the LT2. Analyzing the results of Tulppo et al. (3), it was observed a breakpoint in SD2 very nearby intensities ≈ 80% - 90% VO2MAX during maximal progressive cycling test, having the participants being submitted or not to a parasympathetic system activity blockage before the test. In the present study, HRVT2 showed relative values of speed and HR around 86.1% and 90.5%, respectively. These values are close to intensities related to the second physiological threshold (5, 24).
Despite some criticism, the identification of two physiological thresholds using different variables is possible; due to a cascade of integrated neurophysiological events that occurs in organs and tissues responsible for locomotion (16). In maximal incremental exercises, the first metabolic transition zone matches a significant PNS withdrawal. Concurrently, there are increases in CO2 production derived from the bicarbonate buffering in active muscles at the same time (4, 25). The identification of a plateau in SD1, an index referred to the vagal component of the autonomic balance, has been coincident with the intensity around the first metabolic transition zone (P > 0.05) (3).
From the first physiological threshold during incremental exercises there is an increase in muscle and blood pH levels. Together, there is a greater CO2 production as the exercise progresses, thus intensifying the activation of higher respiratory control centers which leads to an abrupt increase in ventilation minute at the second threshold. Changes in HRV are mediated mainly by alterations in the respiratory sinus arrhythmia and the hyperventilation mechanical effects on the sinus node at this point (26). As a result, there are concomitant changes in respiratory, muscular and CNS systems. In this regard it is important to point out that, regardless of the debate involving physiological thresholds, the present study showed a consistent agreement between different methods identifying physiological thresholds, thus indicating that HRV data set is a valuable tool for physiological assessments used in exercise intensity prescription.
5.2. Reproducibility of the HRV Thresholds
Results of ICC (≈ 0.88; P < 0.001) and Bland and Altman analysis indicated good agreement between test-retest HRV thresholds, with acceptable typical error of measurement between them (≈ 6%). Together with others (7, 8), these results show that thresholds identified by HRV data set, even when analyzing HRV in time domain, is a useful and reliable method to determine the first and second lactate thresholds as identified by LT1 (and LTDmax) and LT2 during maximal running test.
The choice for HRV time domain analysis was based on previous results (27), which reported an unsuccessful spectral analysis in non-stationary HRV data when characterizing the balance between PNS and SNS during exercise. Thus, we used Poincare’s plot after normalization of SD1 and SD2 values by the mean RR, multiplied by 1000. This procedure is similar to that previously reported and allowed us to individualize analysis of the HRV (28).
Furthermore, an important aspect should be considered. Instead of using cycling as the exercise mode, we used running and participants performed a maximal incremental running test on treadmill. Most studies have investigated HRV thresholds in cycling. Therefore, these results are novel because they show that the HRV data set may further identify physiological thresholds during running on treadmill.
Some may suggest that the use of fixed blood lactate concentrations to determine LT1 and LT2, that is 2.0 and 3.5 mmol.L-1, respectively, may be interpreted as a limitation of the present study. Some have criticized the use of fixed blood lactate concentrations as this may provide arbitrary and non-individualized threshold intensities (29). In the present study we first identified LT1 and LT2 individually, by using visual inspection of lactate curves (29). Probably due to a highly exponential lactate-speed relationship, there was a very low agreement between evaluators when determining LT1, having this threshold been identified at intensities much lower (~ 40% SpeedMAX) than those suggested for moderate-well trained middle-distance runners performing maximal running tests, that is between 60% - 70% SpeedMAX (24, 29). Thus, we decided to use fixed blood lactate concentrations to determine LT1 and LT2, Therefore, we acknowledge that fixed blood lactate concentrations may be limited in some situations, however this method provided non-subjective determination of two thresholds in all participants, thus agreeing with the classical widespread threshold conceptual framework for performance and training prescription (29). In fact, both thresholds were identified within the intensity range suggested for LT1 (60% - 70% SpeedMAX or VO2MAX) and LT2 (80% - 90% SpeedMAX or VO2MAX) in moderate-well trained middle-distance runners (5, 24, 29).
On the other hand, we identified the lactate threshold according to the LTDmax in order to determine an individualized threshold. While this method has been considered as a valuable tool to determine lactate thresholds, two aspects should be pointed out. First, this method is considerably susceptible to minimal changes in blood lactate concentration responses over time during exercise, thereby causing “artificial changes” in lactate thresholds sometimes, mainly by fluctuations in lactate values during initial and final stages (30). Second, despite providing a non-subjective individualized threshold, only one physiological threshold can be determined by it so that we could have been unable to properly answer the research question of the present study.
5.3. Conclusions
This study showed that HRV could be a feasible, noninvasive method to determine traditional and new lactate thresholds during maximal running test, given the good agreement between thresholds identified by HRV data set, with those identified by traditional and more recent lactate techniques (LT1 and LT2 and LTDmax). In addition, the HRV thresholds showed good reproducibility between test and retest. Thus, thresholds derived from HVR data set may be a reliable and practical tool to determine traditional lactate thresholds during a maximal running test.