1. Background
Ovariectomy (OVX) is the surgical process of removing the ovaries that subsequently expedites the process of menopause in humans. Both women who are menopausal or post-OVX are characterized by a drop in the concentration of estrogen that can increase fat accumulation in adipose tissue, which can lead to obesity. In addition, its risk factors predispose femalesto dyslipidemia, cardiovascular diseases and some types of cancers (1-7). Specifically, visceral fat is a primary risk factor for metabolic syndrome, which is associated with various other comorbidities such as dyslipidemia, insulin resistance, type 2 diabetes mellitus and hypertension (4, 8-11). Visceral fat deposits can be divided into three major repositories: omental fat surrounding the intestines superficially, mesenteric fat (MES) that is more deeply buried around the intestines, and retroperitoneal fat (RET) which is found on the dorsal side of the abdominal cavity, near the kidneys (10). Additionally, urogenital (URO) is recognized as omental fat in rodents (5).
It is clear in the literature that metabolism is affected by the availability, or lack, of ovarian hormones (8). The attenuation in ovarian hormones, particularly estrogen, promotes deposition and accumulation of fat in adipose, muscle, and liver tissues (11, 12). Fat accumulation in particular body regions in humans (4) and rodents (13) is strongly correlated with hormone concentrations (7). Androgen hormones combined with insulin, growth hormone (GH), or cortisol can positively modulate lipid metabolism. These hormones, both alone and combined, can stimulate lipolysis or lipogenesis in various types of adipose tissue (4). This action is related to the density of androgenic receptors (ARs). For example, visceral adipose tissue has a higher density of ARs. Thus, the decline or even the absence of hormones, such as estrogens can stimulate lipogenesis in visceral adipose tissue.
Adiposity index has been used as a tool for analysis and discussion in the efforts to elucidate the deleterious effects of increased adiposity and possible effects of pharmacological and non-pharmacologic treatments such as physical exercise in humans (14, 15) and rodents (7). Absence of ovarian hormones could lead to increased adipose tissue mass in the body (7, 15) after menopause in humans (16) or OVX animals (7). Fat percentage may also provide information about how lipid metabolism and the organism as a whole are responding to a pharmacological or a non-pharmacological intervention, such as resistance exercise (1, 6, 17).
Previous study showed that resistance training (RT) is capable to prevent the deleterious physiological and metabolic changes promoted by ovarian hormone absence, including increases in abdominal fat and decreases in lipid metabolism (18, 19), increases release of serum TGL from adipose tissue into circulation and reduced total cholesterol levels and atherogenic index in OVX rats (20). In addition, RT has been studied as a non-pharmacological treatment capable of preventing body mass increase (17, 20, 21). This type of exercise can act by altering the metabolism of the body, increasing muscle mass (22, 23) reducing visceral and retroperitoneal adipocyte area in obese rats (21), improving signaling for increased vascularity of adipose tissue, avoiding tissue hypoxia (24). However, to date, studies on lipid content and obesity index in OVX rats submitted to RT are rare. Although it is only speculated that RT prevents fat accumulation induced by OVX, this theory has yet to be definitively investigated in rats.
2. Objectives
The aim of this study was to investigate the effects of RT on adiposity index and the percentage of visceral fat in intact and ovariectomized rats. We hypothesized that RT would reduce the percentage of visceral fat and adipose index, regardless of the ovarian status.
3. Methods
3.1. Animal Care
We analyzed 40 female Wistar rats (Rattus norvegicus var. Albinos, Rodentia, Mammalia) at 13-weeks-old weighting 250 ± 30g, obtained from the vivarium of the Federal University of Sao Carlos (UFSCar), Sao Paulo, Brazil. The rats had free access to water and chow (Labina, Purina, Brazil) and were kept in collective cages in a constant temperature of 23 ± 02°C, and were conditioned to 12-hour dark/light cycles (06:00 am until 06:00 pm). Food intake was not monitored. All procedures were conducted in accordance with the guide of care and handling of laboratory animals in the United States of America (1996). The ethics committee for animal experimentation at the Federal University of Sao Carlos approved the study (protocol no.048/2007).
The composition of the chow diet was: humidity (Max) 13%, Crude protein (Min) 23%, ethereal extract (Min) 4%, fibrous matter (Max) 5%, mineral matter (Max) 10%, Calcium (Max) 1.3%, Phosphor (Min) 0.85% (information provided by the manufacturer).
3.2. Experimental Groups
Rats were randomly assigned to four experimental groups (n = 10): 1) Intact Sedentary (INT-SED) and 2) Ovariectomized Sedentary (OVX-SED), both sedentary groups did not perform RT; 3) Intact resistance trained (INT-RT) and 4) ovariectomized resistance trained (OVX-RT). Both groups assigned to exercise were submitted to 12-weeks of RT.
3.3. Ovariectomy
The ovariectomy procedure was performed when the animals reached 13 weeks of age (20, 25, 26). All animals that underwent surgery had a week of recovery before the onset of RT.
3.4. Resistance Training Protocol: Adopted from Hornberger and Farrar, 2004 (22).
3.4.1. Protocol Familiarization
The rats were exposed to the RT protocol on two separate days with 72 hours (h) of rest between familiarization bouts. The rats were required to climb a vertical ladder (1.1 m; 0.18 m, 2 cm grid, 80° incline) with the unloaded apparatus attached at the proximal portion of the tail by a self-adhesive foam strip. The rat was placed at the bottom of the ladder and familiarized with climbing. When the rats reached the top of the ladder (house chamber) they were allowed to rest for 120 seconds (s). This procedure was repeated until the rats would voluntarily climb the ladder 3 consecutive times, without stimulus (21, 26, 27).
3.4.2. Determination of Maximum Carrying Load
The initial climb was performed with 75% of the animal’s body mass. After the first climb, an additional 30 grams (g) was added on subsequent trials until the rat failed to maintain the ability to ascend the ladder. The highest load successfully carried was considered the rat’s maximal carrying capacity. Failure was determined when the animal could not progress up the ladder after 3 successive stimuli (21, 26, 27).
3.4.3. High-Intensity Resistance Training Session
Resistance training consisted of four ladder climbs with 50, 75, 90 and 100% of the rat’s previously determined maximal carrying load. If the rat reached 100% of the carrying load, an additional 30 g was added, then the rat performed 5 extra climbs at the new maximum. The rest period between each climb was 120 seconds and recovery was set at 72h between sessions (21, 26, 27).
3.5. Experimental Procedure
After the experimental period, rats were euthanized by guillotine. The white adipose tissue deposits (RET, MES, and URO) extraction was performed according to the previously reported anatomical descriptions (3, 5), weighed and stored at -80°C for further analysis.
3.6. Visceral Fat Deposits (PADS)
The RET, MES and URO tissues were dissected by one investigator and weighed immediately after dissection to avoid weight loss. The RET fat pad was taken obtained from the deposit behind each kidney along the lumbar muscles. The MES fat pad consisted of adipose tissue surrounding the gastrointestinal tract from the gastroesophageal sphincter to the end of the rectum. Special care was taken in distinguishing and removing pancreatic cells from MES tissue. The URO fat pad included adipose tissue surrounding the kidneys, ureters, and bladder as well as ovaries, oviducts and uterus (5).
3.7. Determining Percentage Fat
The percentage of lipid for each fat pad was determined by the gravimetric method and the values expressed in percentage of fat (28, 29). The gravimetric method (30) consisted of separating the adipose tissue samples (~ 1 g), digesting the sample in 3 mL of KOH (30%) at 70°C for 10 - 15 minutes. This process was followed by the addition of 3 mL of ethanol and continuous heating at 70°C for 2 hours in a water bath for saponification of lipids. After cooling, 2mL of sulfuric acid (12 NH2SO4) was added. Lipid extraction was performed in three, 10 minutes steps via stirring with the addition of 10, 8 and 6 mL of petroleum ether, respectively. The petroleum ether and combined extracts were washed with 2 mL of distilled water and placed in scintillation vials previously weighed and taken to the laminar flow cabinet to dry. After drying, the vials were weighed to calculate the percentage of fat tissue, as follows: percent body fat (% = [final weight of the scintillation vial (g) - weight of empty scintillation vial (g) x 100]/weight of tissue sample used (g)). Further, the percentage of fat from visceral fats collected was determined: amount of % fat = RET + MES + URO). The difference between of amount of % fat between experimental groups was determined (Difference % of fat =% of fat group A - % of fat group B) (Table 1).
Abbreviations: INT-RT, trained intact; INT-SED, sedentary intact; MES, Mesenteric; OVX-RT, trained ovariectomy groups; OVX-SED, sedentary ovariectomy; RET, Retroperitoneal; URO, Urogenital. Values are presented as means ± SEM (P < 0.05), (n=07).
aSignificant difference from INT-SED.
bSignificant difference from OVX-SED.
cSignificant difference from INT-RT.
3.8. Adiposity Index
Adiposity index is presented as a percentage and was determined as the ratio between the sum of the mass of intra-abdominal and subcutaneous divided by the total body mass (BM) and multiplied by one hundred: adiposity index (%) = (∑ (fat depots)/BM) X 100) (7).
3.9. Statistical Analysis
All data are expressed as means ± standard deviation. SPSS version 17.0 (Somers, NY, USA) software was used for the following analyses. A Shapiro-Wilk test determined that the dependent variables were normally distributed. The homogeneity of variance between groups was assessed by Levene’s test for equality of error variances homoscedasticity. A two-way ANOVA was used to determine the effect of groups (INT x OVX and RT x SED) on MES, RET, URO, fat percentage, and adiposity index, followed by the Post-Hoc-Tukey test (P < 0.05).
4. Results
4.1. Body Mass
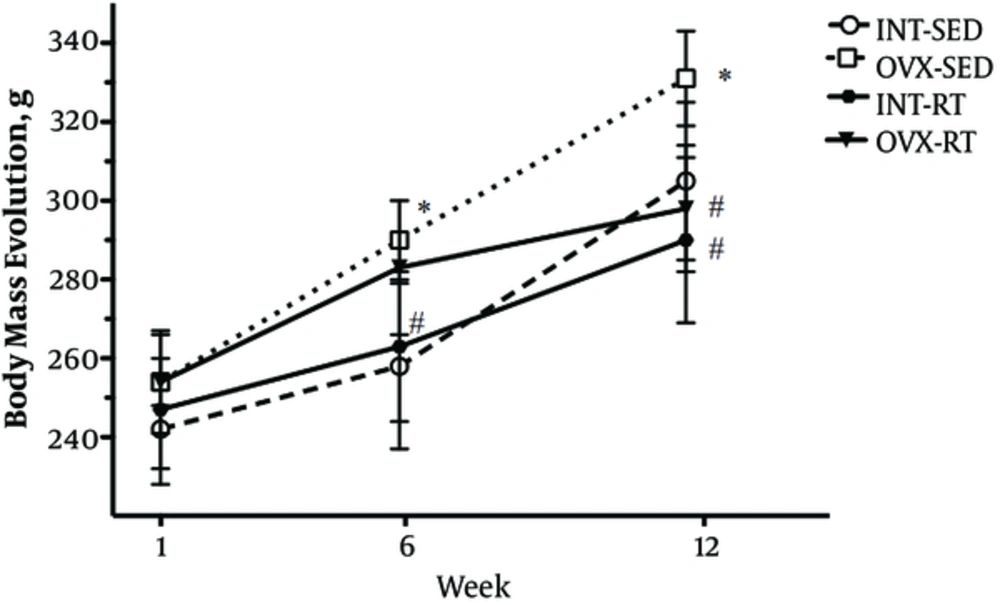
The initial BM did not differ statistically between groups, demonstrating homogeneity at the study genesis. The OVX intervention showed to increase BM in OVX-SED rats, compared to INT-SED rats at the beginning of the 6th week (P = 0.01) and was maintained until the end of the experimental period in the 12th week (P < 0.05). There was a statistically significant interaction between the interventions (RT and OVX status). Post hoc pairwise comparisons using correction revealed that RT lowered BM of the OVX-RT group compared to OVX-SED when measured in the 12th week (P = 0.012). Interestingly, there was no difference in BM between OVX-RT rats compared to INT-RT rats, indicating maintenance of BM in OVX animals throughout the entire experimental period (Figure 1).
4.2. Adipose Tissues
The relative mass of the RET and MES tissue in the OVX-SED was higher compared to INT-SED (P < 0.05). On the other hand, the absence of ovarian hormones, by OVX, did not exert any effects in the mass of URO tissue. Retroperitoneal tissue (RET) mass was lower in the INT-RT compared to INT-SED. However, the mesenteric (MES) and urogenital (URO) tissues remained unaffected by the RT in both, INT-RT and OVX-RT groups. There was a statistically significant interaction between the interventions (RT and ovarian status) in RET and MES tissues. Tests for simple effects revealed that the OVX-RT animals had lower relative mass of RET and MES tissues compared to OVX-SED (P < 0.05). However, URO was not statistically different between groups. The RET mass in the OVX-RT was higher compared to INT-RT (P < 0.05). A similar analysis revealed that MES mass values did not differ between INT-RT and OVX-RT groups. There were no differences in relative URO tissue mass between groups (Table 2).
Abbreviations: INT-RT, trained intact; INT-SED, sedentary intact; MES, Mesenteric; OVX-RT, trained ovariectomy groups; OVX-SED, sedentary ovariectomy; RET, Retroperitoneal; URO, Urogenital. Values are presented as means ± SEM (P < 0.05), (n=07).
aSignificant difference from INT-SED.
bSignificant difference from OVX-SED.
cSignificant difference from INT-RT.
4.3. Percentage of Fat
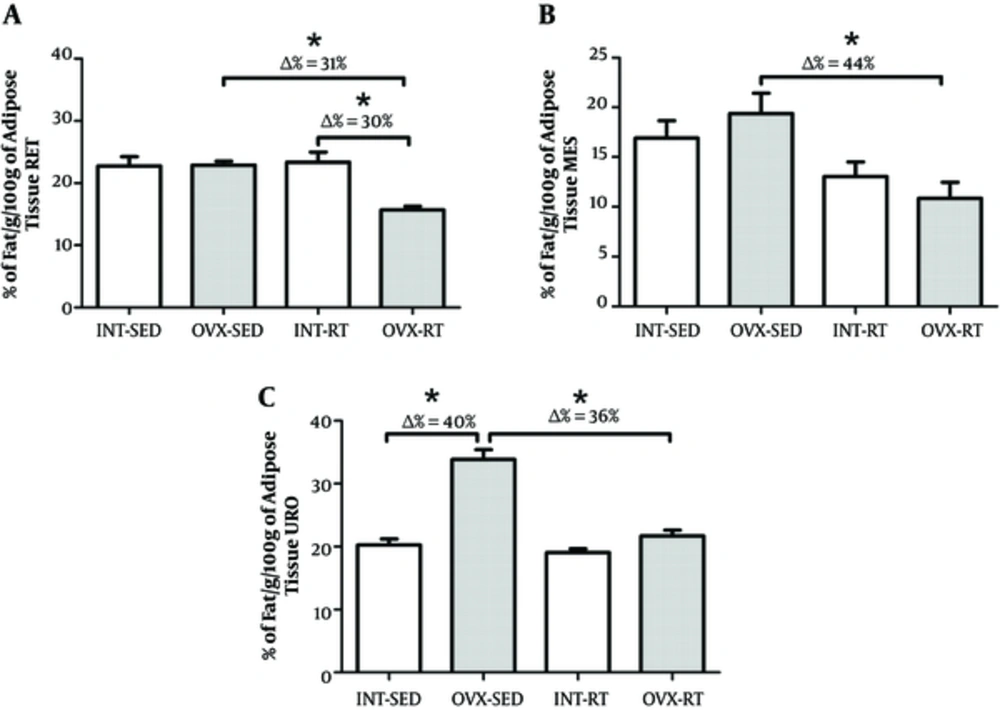
There was no difference in RET and MES tissue between the OVX-SED and INT-SED groups (Figure 2A and 2B). However, the percentage of fat in the URO depot was lower in INT-SED compared to OVX-SED (P = 0.001). A secondary analysis revealed that the change (Δ) in percentage of URO fat between INT-SED and OVX-SED was 40% (Figure 2C).
There was not a significant interaction between the interventions (resistance exercise vs ovarian status) when assessing percentage of fat. Further, there was no difference in MES and URO fat percent between OVX-RT and INT-RT (Figure 3B and 3C). The percentage of RET fat was approximately 30% lower in the OVX-RT group compared to INT-RT (P = 0.001; Figure 2A). There was a statistically significant interaction between the interventions (resistance exercise vs ovarian status) when regarding percentage of fat. The OVX-RT showed lower fat percentage for RET (Δ = 31%), MES (Δ = 44%) and URO (Δ = 36%) compared to OVX-SED (P = 0.001, P = 0.002 and P < 0.001, respectively; Figure 2A and 2B).
4.4. Adiposity Index
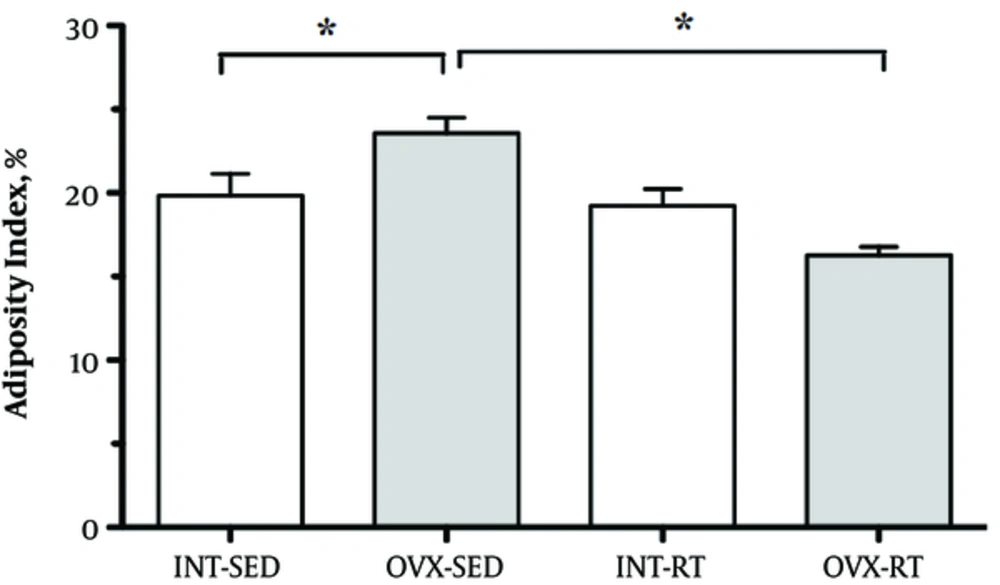
The adiposity index (%) was higher in the OVX-SED compared to INT-SED (P < 0.05) and lower in OVX-RT compared to INT-RT (P < 0.05), showing a statistically significant interaction between the interventions (resistance exercise vs ovarian status). Adiposity index did not differ between INT-RT and INT-SED groups (Figure 3).
4.5. Difference of Percentage (%) of Fat
When we combined values of fat depots % (RET+MES+URO), we observed a greater amount of fat in the OVX-SED compared to INT-SED (~ 22%) and OVX-RT (~ 38%) (P < 0.001). Regarding this variable, we observed a statistically significant interaction between the interventions (resistance exercise vs ovarian status). Rats exposed to RT and OVX decreased % of fat by approximately 15% compared to INT-RT group (P = 0.013). However, the difference was 6% lower in the INT-RT compared to INT-SED (P = 0.269) (Table 2).
5. Discussion
The metabolism of vital organs is affected by the status of ovarian hormones (8). As supported by the current data, the decrease in ovarian hormones, particularly estrogen, promotes the deposition and accumulation of fat in adipose tissue and other organs (12, 13) during menopause in women (4, 8), or after OVX in rodents. These effects can stimulate changes in body composition due to the increase of adipose tissue with concurrent sarcopenia, ultimately predisposing women to obesity (6, 12, 17, 31-34). As such, exercise has been widely recommended as a non-pharmacologic intervention to prevent and mitigate the physiological changes caused by menopause (1, 32).
The purpose of this study was to analyze the influence of RT on fat deposition in visceral adipose tissue by evaluating the adipose index and percentage of fat in INT and OVX rats. The main finding is that RT is capable of decreasing fat accumulation in different fat depots (RET and MES) as well as diminishing the adiposity index and the total fat tissue in OVX animals. These findings provide evidence that RT is a potent intervention capable of preventing body fat gain after OVX. Therefore, it stands to reason that RT interventions could reduce or prevent many of the deleterious effects associated with menopause and obesity.
Exercise can result in reducing the risks of developing obesity in post-menopausal women (35). Similar to previous literature, the results of this study indicate that RT is capable of controlling unwanted BM gain after menopause (17, 36, 37). In this study, the RT was able to prevent BM gain in the OVX group despite the absence of ovarian hormones.
In this study, RT minimized the accumulation of fat in the studied tissues among the INT and OVX animals. According to previous studies (6, 17), RT reduces fat accumulation of adipose tissue in the liver, after a period of food restriction in OVX rats. Accordingly, there is increasing evidence that RT stimulates lipid oxidation in the liver due to the activation of AMP-activated protein kinase (AMPK) (6, 35, 38) and down regulation of lipogenic gene expression by liver enzymes (39).
Resistance training appeared to reduce the percentage of RET, MES and URO fat in the INT and OVX groups, compared to sedentary groups. Interestingly, OVX-RT animals had a greater benefit among the rats exposed to RT, with a decrease of 31% of the fat in the RET tissue compared to INT-RT. A similar response was noted for MES tissue, where the OVX-RT reduced MES content by 44%. Unlike RET and MES, the relative mass of the URO fat depot did not change as a result of RT in either group. However, the percentage of this fat depot was negatively affected by OVX status, where OVX-SED rats increased URO fat by about 40%. Based on these data, we could affirm that a sedentary lifestyle led to an accumulation of fat in the visceral adipose tissue in OVX rats and that RT was able to prevent this gain in OVX-RT rats. Sene-Fiorese et al. (2008) cited that the RET and MES depots are associated with metabolic abnormalities such as dyslipidemia, insulin resistance, type 2 diabetes and even hypertension (28). The rate of catecholamine-induced non-essential fatty acids (NESFA) metabolism accelerates the mobilization of cells in visceral fat tissue due to high rate of lipolysis in adipose cells. Similarly, increased NESFA function affects lipolysis mainly through increased β3-adrenoreceptor function and decreased α2-adrenoreceptor activity. This cascade of events promotes a greater release of NESFA to the portal system, which contributes to the metabolic disturbances observed in obesity. To combat this, exercise expedites lipolysis, especially in MES fat deposits. Although speculative, the current investigation supports this claim that RT may increase the rate of lipolysis, despite the status of ovarian hormone availability (28).
The removal of estrogen in OVX rats decreases the expression of peroxisome proliferator-activated receptor alpha (PPAR-α), a nuclear receptor that controls the genetic programming of the oxidation of fatty acids (40). Our results demonstrated that RT minimized some of the deleterious effects of OVX such as the decrease in percentage of visceral fat tissue in RET and MES tissues. However, the relative mass of URO fat tissues showed no change in body fat percentage, probably due to its protective function associated with reproduction, even after ovariectomy. It should be noted, however, that RT decreased the percentage of fat in OVX animals.
According to Paquette et al. (2008) (39), the expression of factors related to transcription and regulation of hepatic lipids, (PPAR-α, sterol regulatory element binding protein-1c ([SREBP-1c], and stearoyl-CoA desaturase-1 [SCD-1]), decreased lipid oxidation when exposed to downregulation of PPAR-α expression of mRNA and an upregulation of SREBP-1c and SCD-1 in OVX rats. Furthermore, exhaustive exercise decreased SCD-1 in rat liver (41), therefore setting the stage for increased lipolysis. Although unsubstantiated, one may argue that RT can induce activation of AMPK by decreasing the expression of transcription factors related to lipogenesis, increasing the oxidation of fat in the liver, and ultimately reducing the fat content in the liver as discussed previously by Lavoie et al. (2006) (38).
The literature shows that endurance training after OVX decreases visceral fat depots in OVX rats (6, 17). In a study published by our group (20), the state of ovarian hormones exerted a strong influence on the accumulation of fat, as evidenced by the increase of lipid content in liver and fat depots in OVX rats. Data from this study show that a RT protocol was able to partially reduce the lipid content of fat depots, even in the absence of ovarian hormones. Moreover, RT is associated with decreased risk factors for cardiovascular disease, even when no significant body fat loss is observed. Resistance training has proven effective in increasing desirable high-density lipoprotein (HDL-c), lowering low-density lipoprotein (LDL-c), and triglycerides (TGL), along with improving insulin sensitivity and reducing plasma glucose. There is also a reduction in systolic and diastolic blood pressure after RT (42). As such, the value that RT brings to cardiovascular health remains in the absence of fat loss.
Alone, OVX had a negative impact on adiposity index. However, animals subjected to OVX-RT were protected to this rise in adiposity index. Moreover, OVX increased BM (OVX-SED) whereas RT mitigated and even reversed the BM gain in OVX-RT animals allowing for the maintenance of BM similar to INT-SED. It is not surprising that animals in the OVX-SED group increased BM. A previous study (7) showed that mice that underwent OVX had a resulting increase in adiposity index prior to intra-abdominal estrogen injection that offset this change in BM. The data in the present study supports that RT reduces adiposity index in OVX animals, possibly by promoting the use of lipid as fuel through the following mechanisms: 1) enhancement of pathways that promote fat oxidation in muscle; 2) inhibition of fat storage (lipogenesis) in adipose, liver, and muscle; and 3) enhanced rates of adipocyte lipolysis.
Previous work from our laboratory found that the absence of estrogen hormones by OVX promoted lower concentrations of serum TGL compared to INT-SED, suggesting a greater deposition of lipids in adipose tissue (20). However, RT appeared to stimulate adipose tissue to release higher TGL concentration into the circulation compared to inactive controls. Moreover, the same work showed that OVX led to an increase in total cholesterol and atherogenic index. These effects were mitigated by RT resulting in cardiovascular protection. Thus, the reduction of % of fat mass and content by exercise, as RT, could promote benefits to reduce the cardiovascular risks in women post-menopausal and post-ovariectomized women.
Observing the sum of the percentage of total fat of all tissues, we observed that the OVX-SED rats accumulate 22% more fat compared to the INT-SED. Nevertheless, RT was able to prevent the accumulation of fat by about 38% in OVX-RT animals. Similar to these reductions, RT also decreased values for combined fat percentage from baseline in the INT-RT group (around 5%) and over 15% for the OVX-RT animals when compared to INT-RT. The increased percentage of fat caused by OVX (about 22%), and the decrease in the percentage of fat with RT (about 38%), indicating that the presented RT protocol was able to generally reduce fat in the measured fat depots in OVX rats. These results found in the proposed animal model (OVX) can also be found in postmenopausal women. The American College of Sports Medicine’s Position Stand (42) recommends individuals seeking to reduce risk for cardiovascular disease to lose a minimum of 10% BM. However, beneficial improvements in these risk factors were also observed when weight loss was as low, between 5% - 10% (42, 43). Toward this end, future research should focus on the minimum amount of body fat reduction necessary to promote health benefits in menopausal women as found in OVX rats.
We acknowledge some limitations of this study. In the present study, the food intake of the animals was not monitored. Nevertheless, ovarian hormone absence, by OVX, can promote increase food consumption in female sedentary rats even submitted to RT protocol compared to sedentary intact and also submitted to RT (44). This effect suggests that OVX can induce hyperphagia (45). On the other hand, Durval et al. (46) observed that OVX does not promote hyperphagia, this being a controversial topic in the literature. Sedentary lifestyle and total energy expenditure are more relevant factors for the gain of body mass than the increase in food intake (46).
5.1. Conclusions
Our study demonstrated that RT was able to decrease the adiposity index and percentage of visceral fat RET, MES and URO tissue of OVX rats. Resistance training may be a beneficial, non-pharmacological treatment of obesity, specifically after ovarian hormone deprivation. Also, RT may be an important alternative strategy for the control of some of the undesirable effects caused by menopause and OVX, characterized by a decrease of ovarian hormones.