1. Background
The autonomic nervous system (ANS) exerts control over the cardiovascular system. The heart’s activity at rest is primarily regulated by the parasympathetic vagal branch of ANS. Heart rate (HR) and stroke volume increase during exercise to meet the metabolic demands of active skeletal muscles of the body. Rapid tachycardia during exercise is due to withdrawal of parasympathetic response followed by sympathetic dominance phase (1). With cessation of exercise, exponential decline in the HR followed by tapering response occur until full recovery happens. This decline in the HR is primarily due to sympathetic withdrawal and subsequent return of vagal activity (2, 3). The heart rate recovery (HRR) depends on many factors such as intensity of the exercise, age and fitness level of the individuals. Faster HRR in individuals indicates cardiovascular protection since it is associated with higher parasympathetic activity (4).
The influence of ANS on the cardiovascular system is also seen by heart rate variability (HRV) as the variation in the beat to beat intervals. HRV is defined as “the capacity of the heart to change the interval between beats when faced with different situations, where these variations are modulated mainly by the ANS” (5). The HRV evaluation at rest, during and after exercise is an innovative approach to study the different underlying physiological control mechanisms of the body reacting to physical activity (6-8). HRV is a well-established non-invasive tool which can be used to study the effect of mental stress on autonomic control of HR (9). Both HRR and HRV are indicators of cardiac health and all-cause mortality in a clinical perspective (4). These measures are also used for exercise prescription and for evaluating the outcomes of exercise training. They also predict aerobic fitness and incidence of overtraining (3, 10, 11).
Acute response indicates decrease in the HRV parameters (e.g. root mean square of successive differences (RMSSD) and high frequency spectral power (HF)) which are vagal related indices after a single exercise bout (12). The restoration of vagal tone has been examined focusing on intensity of exercise (13-15), type of exercise (16, 17), exercise duration (18), recovery duration (19), recovery type (20) and cardiorespiratory fitness (21). The sympatho-vagal balance is indicated by low frequency-high frequency ratio (LF-HF ratio). If this ratio is less than 1, there is parasympathetic predominance, whereas, ratio more than 1 reflects sympathetic predominance (9).
Soccer is the most known game in the world. Soccer players’ average intensity of work during match is generally between 80% -90% of HRmax which consist of walking (25% of total work), jogging (37%), sprinting (11%), backing (6%), and cruising (20%) (22). Field hockey resembles soccer in terms of physical and physiological demands of play, where the player’s average velocity has been estimated to range between 2.2 - 2.59 m/s and maximal (temporary) velocity up to 8.03 - 9.27 m/s (23). It has been reported that field hockey players spend most time in walking (46.5%), jogging (40.5%), standing (7.4%) and sprinting (1.5 %) during a match play (24), which reflects a slight difference in overall intensity compared to soccer. However, both sports largely utilize the aerobic system interspersed with short anaerobic activities. On the other hand, basketball is a game that demands intermittent bouts of high intensity. Drinkwater et al. found that in international level games, players reached up to 95% of their HRmax (25). Basketball players have been reported to spend 34.1% of the game time in running and jumping, 56.8% in walking and 9% in standing (26), making it a predominantly anaerobic sport. This difference in the physiology of these sports is expected to reflect in the autonomic function of athletes, as a result of exposure to long-term sport-specific drills.
Previous studies have examined the effects of sports activity (27) and competition (28, 29) on vagal-related HRV parameters in soccer players. Its use as a measure of performance in soccer has also been explored (30). Only one study to date has assessed cardiac autonomic response along with perceived tiredness to competitive overload in field hockey players (31). Esco and Willifiord compared cardiovascular autonomic modulation in basketball players with active healthy individuals (32). Migliaro et al. compared young sedentary, young non-sedentary (soccer and basketball players) and old sedentary individuals on RMSSD, HF and low frequency (LF) spectral of HRV (33). A body of knowledge exists comparing the somatotype, physical and psychological characteristics of soccer, field hockey and basketball players (34-38). However, the comparison of autonomic function across soccer, field hockey and basketball athletes warrants investigation.
2. Objectives
The purpose of our study was to evaluate the HRR and HRV parameters in collegiate male soccer, field hockey and basketball athletes.
3. Methods
3.1. Participants
Fifty-five male collegiate athletes (20 soccer players, 18 field hockey players and 17 basketball players) were recruited from M. A. K. Pataudi Sports Complex, Jamia Millia Islamia, New Delhi, India. Subjects between the age of 18 - 25 years, with BMI (body mass index) ranging from 18.9 - 24.9 kg/m2, indulging in sport-specific training for at least the last 3 months (4 - 5 days/week), and having participated in university level competition, were included in the study. Those with a history of cardiac or respiratory pathology, taking medications that alter cardiac response or recovery, diagnosed of a chronic health condition, smokers and alcoholics, were excluded. All participants gave their written consent, after verbal explanation of the procedure, risks involved and participation being entirely voluntarily. All uncertainties among the participants regarding the procedure were cleared. Prior to the experimental procedure, ethical approval for the study was granted from the institutional ethics committee, Jamia Millia Islamia, New Delhi, India.
3.2. Instrumentation and Data Acquisition
Measurements were undertaken in a quiet room, at a temperature between 24 to 30°C and at the same time (between 10:00 am and 1:00 pm) during the day to nullify the effect of circadian rhythm on HRV. Skin was prepared by wiping with alcohol swab for the electrodes placement to reduce impedance. Electrocardiograph (ECG) was recorded at rest and after sub-maximal exercise in supine lying for 10 minutes while breathing approximately at 15 breaths per minute. HRV analysis was done on final 5-minute epochs segment of ECG. ECG data was sampled at 1000 Hz, band pass filtered between 0.3 and 100 Hz, and stored on computer for analysis (Powerlab 8/30 Data Acquisition System with LabchartPro: AD Instrument, Australia). For HRV analysis, R-R intervals were selected by setting threshold at 0.5 mV and ectopics were excluded from analysis by “beat classifier” function of software. HR was recorded by a heart rate monitor (Polar Electro, RS 400, Kempele, Finland) with chest strap.
3.3. Procedure
Subjects were instructed to lie down supine for 10 minutes to attain complete relaxation during which, resting heart rate (RHR) and HRV were recorded. Following this, the participants performed an incremental treadmill test at sub-maximal exercise intensity. A 5 - 10 min familiarization session for treadmill running was given to each participant 24 hours before actual testing. The incremental running test began with an initial speed of 8 km/h with grade 0 and increased 1 km/h every minute until the subject reached sub-maximal target heart rate (THR), experienced adverse signs or symptoms, requested to stop, or experienced any emergency situation. Beat-by-beat HR responses were monitored during the running test. The THR was calculated using Karvonen formula, THR = [(HRmax - RHR) × 80% - 85% Intensity] + RHR, where maximal heart rate, HRmax = 206.9 - 0.69 × age (years).
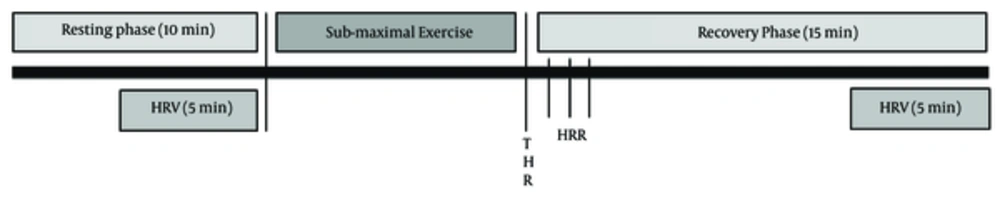
After the exercise protocol, subjects returned to supine lying within 5 seconds. HRR was recorded at 1st, 2nd and 3rd minutes, post-exercise. The subject remained in this position for 15 minutes, during the final 5 minutes subjects were instructed to breathe at a frequency of around 15 breaths per minute. Talking and excessive movement during this time period was avoided. ECG was recorded for 10 minutes, but to ensure being stationary, the final 5 minutes were used to analyze HRV. Time domain HRV measures were obtained by mean RR intervals (meanNN) and the root mean square of successive differences (RMSSD) whereas frequency domain measures of HRV were derived by Fast Fourier Transformation: they were low-frequency (LF; 0.04 - 0.15 Hz) and high-frequency (HF; 0.15 - 0.4 Hz) spectral power. Temporal and spectral measures of HRV have been reported to be reliable and reproducible by previous literature (7, 39) (Figure 1).
3.4. Statistical Analysis
Data were analyzed using SPSS version 21. The Shapiro-Wilk test was used to verify the normality of distribution for all variables. RHR and RMSSD scores that demonstrated non-normal distribution were log transformed for further analysis. The demographic characteristics and baseline measures were compared between the groups using one-way ANOVA. A repeated measure ANOVA was used for HRR. 3 × 2 mixed model ANOVA was employed for meanNN, HFnu to find out main effect of group (soccer, field hockey and basketball) and time (resting, post-exercise) as well as Group × Time interaction. 3 × 2 mixed model ANCOVA was used for RMSSD, LFnu and LF-HF ratio considering baseline measures as covariate. Post hoc Bonferroni test was used for multiple comparisons. Statistical significance level was kept at P ≤ 0.05.
4. Results
Demographic characteristics showed no significant difference between the groups in age, BMI, RHR and THR while significant differences were found in weight (F2,52 = 9.298, P < 0.001) and height (F2,52 = 4.003, P = 0.024). Post hoc analyses showed that basketball players are taller than soccer players and heaviest among the 3 groups (Table 1).
| Participant Characteristics | Soccer Players (n = 20) | Field Hockey Players (n = 18) | Basketball Players (n = 17) | P Value | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | S Vs FH | S Vs B | FH Vs B | |
| Age, y | 21.10 ± 2.12 | 21.44 ± 2.14 | 20.41 ± 1.62 | 1 | 0.899 | 0.394 |
| Weight, kg | 61.33 ± 3.88 | 61.43 ± 4.73 | 67.7 ± 6.33 | 1 | 0.001* | 0.002* |
| Height, m | 1.67 ± 0.05 | 1.68 ± 0.05 | 1.72 ± 0.06 | 1 | 0.037* | 0.07 |
| BMI, kg/m2 | 21.77 ± 1.36 | 21.68 ± 1.03 | 22.62 ± 1.38 | 1 | 0.142 | 0.098 |
| RHR, bpm | 61.6 ± 5.28 | 63.22 ± 4.44 | 65.29 ± 4.08 | 0.869 | 0.060 | 0.586 |
| THR, bpm | 173.1 ± 1.37 | 172.55 ± 1.29 | 173.58 ± 1.17 | 0.598 | 0.768 | 0.065 |
Abbreviations: B, basketball; BMI, body mass index; FH, field hockey; RHR, resting heart rate; S, soccer; SD, standard deviation; THR, target heart rate.
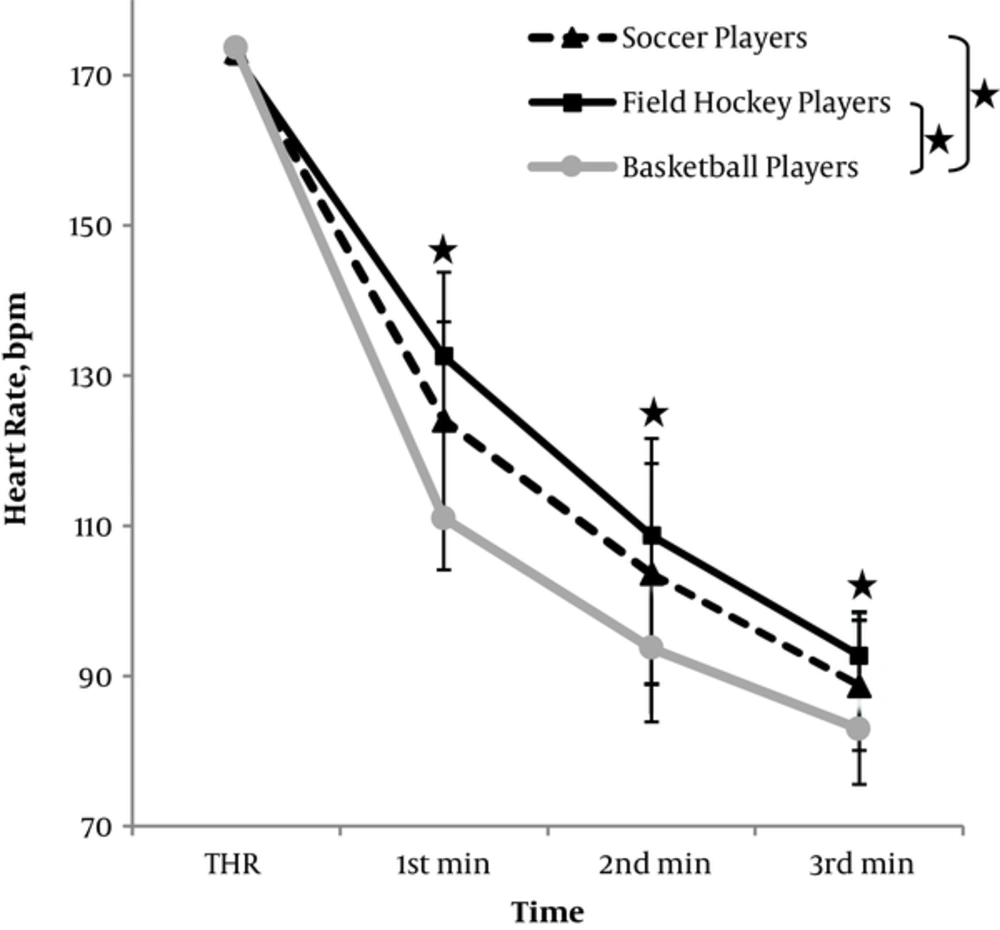
HRR showed significant group effect (F2,52 = 10.34, P < 0.001), time effect (F3,52 = 2012.08, P < 0.001) and group×time interaction effect (F6,52 = 10.72, P < 0.001). Post hoc revealed that basketball players showed faster HRR than soccer (P = 0.017) and field hockey players (P < 0.001) during first 3 minutes of recovery after exercise. Also the decrease in the heart rate is significant in first minute, second minute and third minute in soccer (P < 0.001), field hockey (P < 0.001) as well as basketball players (P < 0.001) Figure 2.
Time and frequency domains of HRV measures of soccer, field hockey and basketball players at rest are presented in Table 2. At baseline, no significant difference was found in meanNN (F2,52 = 1.656, P = 0.201) as well as HFnu spectral (F2,52 = 1.085, P = 0.345) of HRV parameters among the groups. While significant differences were found in RMSSD (F2,52 = 14.715, P < 0.001), LFnu (F2,52 = 4.33, P = 0.018) and LF-HF ratio (F2,52 = 3.168, P = 0.05). Post hoc revealed that soccer players showed higher value RMSSD than field hockey (45.77 %) and basketball players (157.77 %), also field hockey players were higher than basketball players (76.83 %) in that regard. Basketball players showed higher LF-HF ratio than soccer players (43.12 %) and higher LFnu values than soccer (32.23 %) as well as field hockey players (30.64 %) (Table 2). Mixed analysis of variance revealed that there was no significant difference between the groups at post exercise recovery in meanNN (F2,51 = 0.273, P = 0.762), RMSSD (F2,51 = 2.371, P = 0.104), HFnu (F2,51 = 2.194, P = 0.122) and LF-HF ratio (F2,52 = 1.114, P = 0.336) while there was significant difference showed in LFnu spectral power (F2,52 = 7.629, P = 0.001). Post hoc revealed that there was a higher LFnu value in basketball players than soccer players as well as field hockey players (Table 3).
| Participants Characteristics | Soccer Players (n = 20) | Field Hockey Players (n = 18) | Basketball Players (n = 17) | P Value | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | S Vs FH | S Vs B | FH Vs B | |
| HRV time domain | ||||||
| Mean NN, ms | 926.28 ± 180.25 | 853.36 ± 81.14 | 879.71 ± 74.27 | 0.238 | 0.796 | 1 |
| RMSSD, ms | 102.80 ± 46.46 | 70.52 ± 40.85 | 39.88 ± 16.42 | 0.05* | < 0.001* | 0.015* |
| HRV frequency domain | ||||||
| LF, nu | 36.14 ± 12.93 | 36.58 ± 15.45 | 47.79 ± 11.16 | 1 | 0.032* | 0.048* |
| HF, nu | 46.61 ± 11.87 | 41.69 ± 9.83 | 45.35 ± 9.67 | 0.475 | 1 | 0.933 |
| LF/HF | 0.80 ± 0.33 | 0.90 ± 0.42 | 1.15 ± 0.51 | 1 | 0.05* | 0.283 |
Abbreviations: B, basketball players; FH, field hockey players; HF (nu), normalized high frequency spectral power; HRV, heart rate variability; LF (nu), normalized low frequency spectral power; LF/HF, low frequency/high frequency ratio; mean NN, mean RR normal to normal interval; RMSSD, root mean square of successive differences; S, soccer players; SD, standard deviation.
| Participants Characteristics | Soccer Players (n = 20) | Field Hockey Players (n = 18) | Basketball Players (n = 17) | P Value for Group Effect | P Value for Time Effect | P Value for G × T Interaction | ||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | S Vs FH | S Vs B | FH Vs B | |||
| HRV Time Domain | ||||||||
| Mean NN, ms | 0.264 | 0.59 | 1 | < 0.001* | 0.342 | |||
| Baseline | 926.28 ± 180.25 | 853.36 ± 81.14 | 879.71 ± 74.27 | |||||
| Post-exercise | 712.54 ± 73.76 | 694.55 ± 51.41 | 689.72 ± 54.13 | |||||
| RMSSD, ms | 1 | 0.453 | 1 | < 0.001* | 0.237 | |||
| Baseline | 102.80 ± 46.46 | 70.52 ± 40.85 | 39.88 ± 16.42 | |||||
| Post-exercise | 23.38 ± 8.95 | 18.39 ± 8.44 | 16.27 ± 11.23 | |||||
| HRV Frequency Domain | ||||||||
| LF, nu | 0.745 | 0.02* | 0.001* | < 0.001* | 0.001* | |||
| Baseline | 36.14 ± 12.93 | 36.58 ± 15.45 | 47.79 ± 11.16 | |||||
| Post-exercise | 61.64 ± 14.76 | 57.34 ± 11.44 | 73.43 ± 3.90 | |||||
| HF, nu | 0.438 | 0.368 | 1 | < 0.001* | 0.247 | |||
| Baseline | 46.61 ± 11.87 | 41.69 ± 9.83 | 45.35 ± 9.67 | |||||
| Post-exercise | 25.28 ± 10.24 | 23.98 ± 8.08 | 19.82 ± 4.28 | |||||
| LF/HF | 1 | 1 | 0.427 | < 0.001* | 0.336 | |||
| Baseline | 0.8035 ± 0.33 | 0.90 ± 0.42 | 1.15 ± 0.51 | |||||
| Post-exercise | 3.05 ± 1.97 | 2.82 ± 91.73 | 3.90 ± 1.01 | |||||
Abbreviations: B, basketball; FH, field hockey; HF (nu), normalized high frequency spectral power; HRV, heart rate variability; mean NN, mean RR normal to normal interval; LF (nu), normalized low frequency spectral power; LF/HF, low frequency/high frequency ratio; RMSSD, root mean square of successive differences; S, soccer; SD, standard deviation.
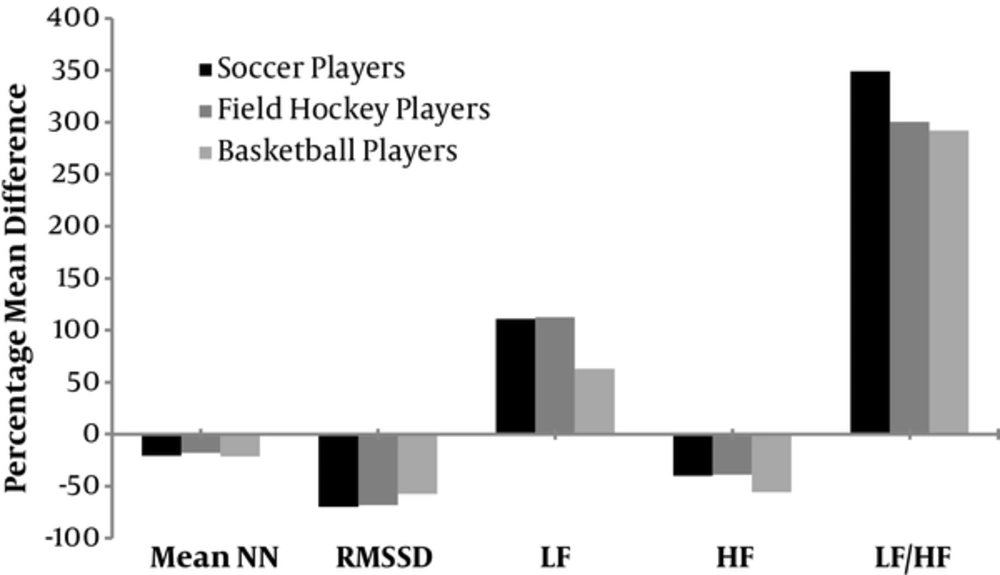
Relative changes in time and frequency domain HRV parameters at baseline and post-exercise are presented in Figure 3. Post-exercise meanNN, RMSSD and HFnu measures of HRV were significantly lower compared with baseline values with F1,52 = 147.29, P < 0.001, F1,52 = 8.329, P = 0.006, and F1,52 = 136.07, P < 0.001 respectively. Post-exercise LFnu and LF-HF ratio were significantly increased during recovery when compared to baseline values with F1,52 = 156.01, P < 0.001 and F1,52 = 18.51, P < 0.001 respectively.
5. Discussion
The purpose of present study was to compare the autonomic control of heart in soccer, field hockey and basketball players at rest and during recovery. Previous studies have reported a more ectomorphic profile in basketball players when compared to hockey players (38). While our study did not explicitly study somatotypes, we found no significant difference between the groups in terms of BMI. The basketball group was found to be the heaviest among all three groups.
5.1. Resting HR and HRV
The RHR of athletes is lesser than the sedentary individuals (29), due to increased vagal tone that causes resting bradycardia which is a cardiovascular adaptation to endurance training (40). This study revealed similar RHR between the groups, indicating comparable physical fitness.
Previous studies have shown that training load significantly influences the cardiovascular autonomic activity control (21). Bricout et al. reported lesser LFnu and LF-HF ratio during rest in soccer players that increased with physical and psychological stress (27). Parrado et al. found higher value of LF-HF ratio in field hockey players showing changes in cardiac autonomic response during World Cup (31). HF indices of HRV more accurately indicate parasympathetic dominance, while RMSSD is shown to be less affected by the respiration at rest (41). RMSSD which is indicative of vagal activity was found to be significantly lower whereas, LFnu that reflects sympathetic activation was significantly higher in the basketball group. LF-HF ratio that represents sympatho-vagal balance related to arterial baroreflex activity and cardiovascular stresses was also shown to be significantly elevated. These suggest an enhanced sympathetic and depressed parasympathetic tone at rest, in basketball players, relative to soccer and field hockey athletes. While soccer and hockey athletes were comparable in terms of HRV indices, significant differences in the profiles of basketball players were recorded.
5.2. Heart Rate Recovery
Studies that have examined the relationships between HRR and physical activity, suggest that faster HRR is associated with good physical fitness (3, 42). The current study revealed faster HRR in the basketball players than soccer players and field hockey players. The difference found in the HRR in the first minute was about 62 beats in basketball players while for soccer and field hockey players it was about 49 beats and 40 beats respectively. This showed that the rapid change in autonomic control with sympathetic withdrawal or increased parasympathetic activity after cessation of exercise in basketball players was higher relative to soccer players and field hockey players. This could be attributed to the frequent, short-burst, sprinting activity in basketball. Faster HRR has been shown to be associated with sprinting (43). With the repeated bouts of high intensity activity and recovery bout over a period of time, adjustments in ANS could be expected, that might control the activity of heart via return of vagal tone. Studies have shown that athletes who engage in intermittent endurance sports have faster HRR than athletes involved in continuous endurance sports after maximal exercise (44). Also, previously found 1 min HRR to be about 40 beats in the first minute after exercise for soccer players as well as in sedentary during the competition (29). Although our study was performed during the competitive season, level of competition could be the reason for the difference because the participants were in the middle of intra-university competition.
5.3. Post-Exercise HRV
This study incorporated a submaximal exercise followed by 15 minutes of recovery in supine showing altered autonomic control post-exercise. RMSSD (69.79% in soccer players, 67.96% in field hockey players and 57.41% in basketball players) and HFnu indices (40.09% in soccer players, 38.58% in field hockey players and 55.48% in basketball players) of HRV showed significant reduction after 15 minutes of recovery when comparing with the baseline values; indicating lesser parasympathetic activity. We also found elevated LFnu (110.58% in soccer player and 112.73% in field hockey players and 62.98% in basketball players) and LF/HF ratio post recovery increased sympathetic outflow to the heart. The difference in resting and post-exercise values of HRV signifies that the shift in autonomic modulation during exercise did not return to resting levels. The findings are consistent with previous studies that emphasize parasympathetic withdrawal and increased sympathetic tone elicited during activity. Moreover, as demonstrated by Barak et al., Terziotti et al., Parekh and Lee, and Kamath et al. the recovery following activity was not completed in 15 minutes. Both the depressed vagal and elevated sympathetic tone failed to return to resting levels within the recovery period following exercise (14, 15, 20, 45).
The results of post-exercise HRV demonstrated an overall reduction in the indices of vagal activity and increase in the markers of sympathetic tone. This is expected, as the transition from rest to exercise entails parasympathetic withdrawal and sympathetic up regulation in order to raise the metabolic rate and meet the energy demands of physical activity. This elevated sympathetic outflow and reduced parasympathetic reactivation even after 15 minutes of exercise recovery could be due to the following i) increased level of metabolites, ii) elevation of catecholamines and other sympathetic transmitters and iii) arterial baroreflex stimulation during the exercise (46-48).
The magnitude of recovery (difference of resting and post-exercise HRV) was similar between the 3 groups for all variables except LFnu. LFnu which denotes sympathetic activity was found to be increased in all groups post-exercise but the increase was significantly greater in the basketball group. These sports have different aerobic and anaerobic contribution during the game, the player’s effort during the game, found to be 70% of the player’s effort in a field hockey game, 80% of player’s effort in a soccer and 50 % in the basketball in terms of aerobic demands (49-51). The aerobic fitness shifted the autonomic power distribution more towards parasympathetic activation (52). Moreover, increased sympathetic dominance in basketball players can be due to increased metabolites as a consequence of repeated anaerobic bouts of activity. Further changes in HRV might not be explained merely by the reflex mechanism, but weakening of baroreflex activity influence on post exercise autonomic control over cardiovascular activity.
Thus, it is reasonable that the current study explained the difference in the autonomic contribution in these different sports and gives cardiovascular fitness profile of athletes. This information is useful for incorporating different training methodologies or development of a specific training program for cardio-respiratory fitness in athletes. There was some limitation in the study. The type of training and amount of training done by the participating athletes was not fully explained in the study, although the training load is somewhat included into the inclusion criteria. Also, there was greater relation of HRV measures to the training load in the athletic population. Further research can be focused on considering the physiological (primarily aerobic and anaerobic) and psychological characteristics in these athletic groups.
In conclusion, the results of the present study suggest that basketball players exhibit greater sympathetic outflow, at rest and following activity. The autonomic profiles of soccer and field hockey were found to be comparable.