1. Background
The use of anabolic-androgenic steroids (AAS) among physical activity practitioners has increased in the last years, and it is associated with body image disorders and clinical complications, including kidney disease (1-3). In Brazil, the estimated prevalence of AAS use among recreational physical activity practitioners varies from 2.1% to 31.6% (4).
Physical activity, although indicated to achieve a good health condition and prevent many chronic diseases, including cardiovascular diseases, can be associated with substance abuse and consequent development of organ dysfunction (5, 6). Past studies suggest an increased risk of developing chronic kidney disease among AAS users, and it can be asymptomatic for a long time before diagnosis (7, 8). In recent years several studies have been conducted to early identify kidney diseases through the use of new biomarkers (9-11), but it has not been yet investigated in the setting of AAS use. The aim of the study is then to investigate new kidney injury biomarkers among bodybuilders using AAS.
2. Methods
This is a cross-sectional study with 57 individuals, regular physical activities practitioners, randomly selected from fitness centers in the city of Fortaleza, Northeast Brazil, in the period from April to July 2016. The protocol of this study was reviewed and approved by the ethics committee of the university of Fortaleza (protocol number 1.028.465/2015).
The studied individuals were divided into two groups: AAS users and non-users. Inclusion criteria were: age ≥ 18 years-old, physical activity practice for more than 3 months; AAS use in the last 2 months (AAS users) and AAS “never used” (for non-users). Individuals with previous history of kidney disease, use of non-steroidal anti-inflammatory or other nephrotoxic drugs and any other condition that could affect kidney function were excluded.
After a first contact, explaining the purpose of the study and signing an informed consent, recruited participants were scheduled for a medical interview and collection of blood and urine samples. The following laboratory tests were done: serum creatinine, monocyte chemoattractant protein-1 (MCP-1), kidney injury molecule 1 (KIM 1) and cystatin C. Glomerular filtration rate (GFR) was estimated by the CKD-EPI equation (12). Exercise intensity was assessed through the Borg scale (13).
Statistical analysis was done with the SPSS program version 20.0 (IBM, USA). Normality tests were done, student t test and Man Whitney tests were done when appropriate, as well as Anova, Kruskal Wallis and chi square tests. Investigation of correlation was done with Pearson’s test. Significance level was set on 5% (P < 0.05).
3. Results
A total of 57 individuals were included, 28 in AAS users group and 29 in non-users. AAS users’ mean age was 26 ± 5 years, while in non-users it was 31 ± 9 years (P = 0.006), and there was a predominance of males in both groups (89.3% in AAS users, 55.2% in non-users). Exercise intensity was higher in AAS users (7.9 ± 1.7 vs. 6.5 ± 2.2, P = 0.01).
Caffeine was the most frequently used stimulant (71.4% in AAS users, 24.1% in non-users, P < 0.001). AAS users reported a frequent use of stimulating substances before training (6 days a week). The most frequently used AAS were testosterone (89.3%), boldenone (50%) and stanozolol (46.4%).
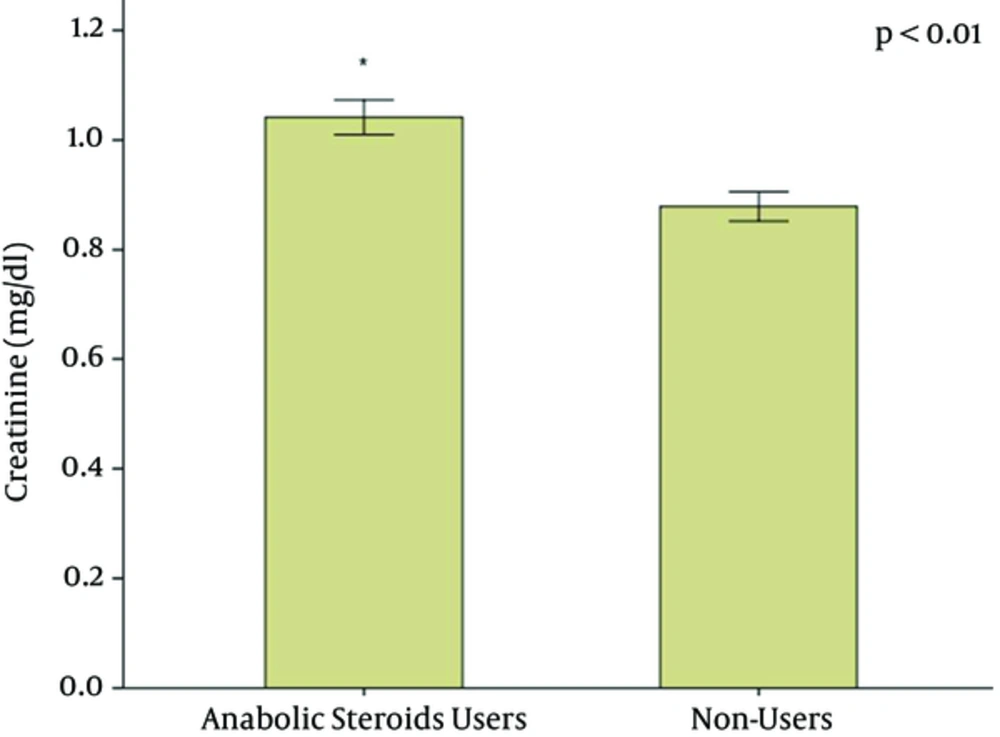
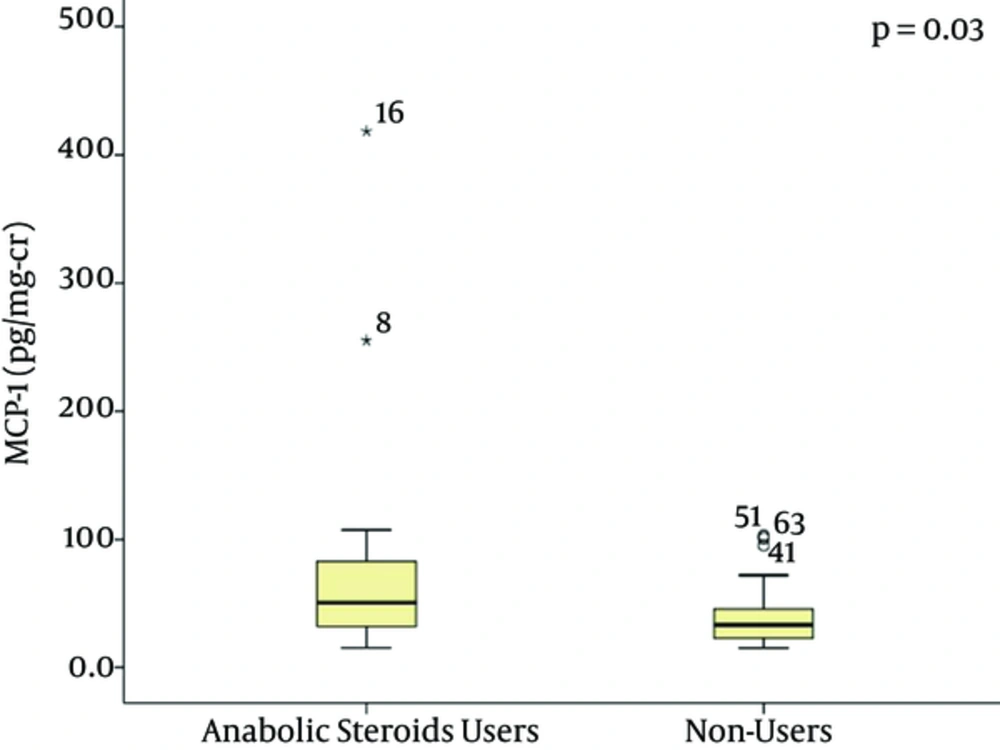
Laboratory tests showed a higher creatinine level in AAS users (1.04 ± 0.17 mg/dL) in comparison to non-users (0.88 ± 0.14 mg/dL), P < 0.001 (Figure 1). Mean GFR was similar in both groups (98 ± 17 vs. 105 ± 13 mL/min/1.72 m2, P = 0.101). Urea levels were similar in both groups (33 ± 9.5 mg/dL vs. 29.8 ± 6.3 mg/dL, P = 0.141. Median levels of MCP-1 pg/mg-creatinine were higher in AAS users (50.66 pg/mg-creatinine, range 31.99 - 255.25 pg/mg-creatinine vs. 33.26 pg/mg-creatinine, range 22.82 - 102.98 pg/mg-creatinine, P = 0.039), as illustrated in Figure 2. KIM-1 levels were not significantly different between the two groups (0.47 ± 0.34 ng/mg-creatinine vs. 0.69 ± 0.47 ng/mg-creatinine, P = 0.065). Cystatin C levels showed a tendency to be higher in AAS users (0.64 ± 0.46 mg/L vs. 0.43 ± 0.36 mg/L, P = 0.059).
4. Discussion
Kidney disease has been reported among AAS users, but only in individuals with significant kidney damage (3, 8). The discovery of novel biomarkers has a great importance for early detection of kidney disease and allows the adoption of measures to prevent or slow kidney disease progression. To our knowledge this is the first report on novel kidney injury biomarkers among AAS users. Our study evidenced a significant increase in the levels of novel biomarkers among AAS users with no clinically evident disease, which illustrates incipient kidney injury.
The majority of AAS users were young males, with no comorbidity, and abnormalities in renal function biomarkers demonstrates their risk for kidney disease development. The AAS and vitamin supplements market is increasing in the whole world, which was boosted by the increasing number of people practicing physical activity and looking for better results. Most stimulants have caffeine and are used almost daily due to severe requirements during training, and the long-term effects of these substances are yet not completely known (14).
Previous studies show that a great part of AAS users combine different steroids, usually in higher doses than normally recommended and for a longer time (2, 15), which makes research in this field important to better know the effects of this practice. The most used substances are nandrolone and testosterone, probably due to their low cost (16).
There are few studies investigating the clinical manifestations of physical activitiy practitioners using AAS (17). Hypertension, anxiety and sexual dysfunction are the main reported manifestations (18). Hypertension, associated with dyslipidemia, can be found in AAS users, and this can be a result of hyperactivation of adrenal cortex and rennin secretion increase (19). Whether this can be associated with kidney disease is still a matter of research.
Renal function abnormalities are described among AAS users, usually when supra-physiologic doses are used, including the use of veterinary supplements (3, 8). One of the mechanisms that have been proposed for kidney injury in these cases is the excess use of vitamin D, leading to hypercalcemia, hypercalciuria and nephrocalcinosis (8, 20). These patients are at high risk for developing chronic kidney disease and then the early detection of renal damage is crucial to prevent disease progression. For this purpose there are novel biomarkers that have been investigated here.
The oldest and most used renal function biomarker is creatinine, which is influenced by muscular mass and protein ingestion. It is also secreted by renal tubules, which makes this a non-ideal biomarker. Creatinine increase has been identified in patients using boldenone and creatine supplement (21). In the present study there were also higher creatinine levels among AAS users in comparison with non-users. Cystatin C, although considered a slightly better renal function biomarker than creatinine (22), did not show significant difference when comparing AAS users vs. non-users. The levels of the new biomarker MCP-1 were significantly higher among AAS users in our study, suggesting subclinical renal inflammation, which can be directly caused by AAS use, including the possibility of chronic interstitial nephritis. Kidney biopsies from patients who have used anabolic steroids plus vitamin supplements and developed acute kidney injury have shown interstitial nephritis and acute tubular necrosis (8). Renal function monitoring with new biomarkers could be important to detect early and subclinical changes in AAS users and prevent further kidney damage, such as that demonstrated in previous case reports (8, 20).
These renal abnormalities could be a consequence of oxidative stress caused by AAS use, which involves fibrosis, cellular proliferation, cytokine release and other substances that in turn allow the increase of some biomarkers, including MCP-1 and KIM-1, making it possible to detect early kidney damage (23).
In summary, we have identified subclinical kidney injury among AAS users, mainly through MCP-1 increase. Although the exact mechanism of AAS-associated kidney injury remains to be elucidated, there is evidence that these substances, along with several other supplements, are nephrotoxic and its use can cause severe kidney disease, as previously reported. Continuous renal function monitoring and early detection of kidney injury is very important for AAS users, and more important than this is patient counseling to avoid use of these substances, unless otherwise prescribed for treatment of some medical condition.