1. Background
The activity of public speaking, typical of the University professor, is associated with increased blood pressure (BP) and a risk of developing systemic hypertension (1), thus, the control of BP and the sympathetic-vagal balance are important factors in reducing this risk (2). The protective effects of resistance exercise (RE) on the cardiovascular systems of healthy individuals and individuals affected by diseases have also been highlighted (3). Umpierre and stein (4) propose RE as a possible strategy for cardiovascular disease prevention and rehabilitation.
Ribeiro et al. (5) investigated the importance of the aerobic exercise on the blood pressure responses of University professors. Besides, other authors investigated the post-exercise hypotension (PEH) phenomenon, which is characterized by a reduction in BP, within normal limits, to values below those observed in the pre-exercise rest period (6), and its duration may occur up to 24 hours (24h) after the exercise session (7). However, there are few studies (7-11) that observe the BP responses following a single RE session for an extended period (24h post-exercise periods), especially at different intensities of RE in University professors.
Studies using ambulatory BP monitoring (ABPM) (7-11) can contribute to a better explanation of the PEH phenomenon for more prolonged acute periods of daily life and at important periods, such as when awake and during sleep. The ABPM can also be useful to demonstrate periods of BP reactivity during work activity, along with reduced nocturnal dipping of BP during sleep, similar to what occurs in University professors after a controlled period without the prior execution of aerobic exercise (5). Both work activity and reduced nocturnal dipping of BP are related to increased cardiovascular risk (1, 12, 13). Therefore, analyzing the BP responses 24h after RE at different intensities, which suggest different cardiac autonomic modulations during the post-exercise periods (14, 15), can provide important information, particularly about University professors, who are subject to high daily levels of stress (16, 17).
2. Objectives
The objective of this study was to investigate the hemodynamic and cardiac autonomic responses of University professors during teaching and sleeping periods after RE sessions of different intensities.
3. Methods
3.1. Sample
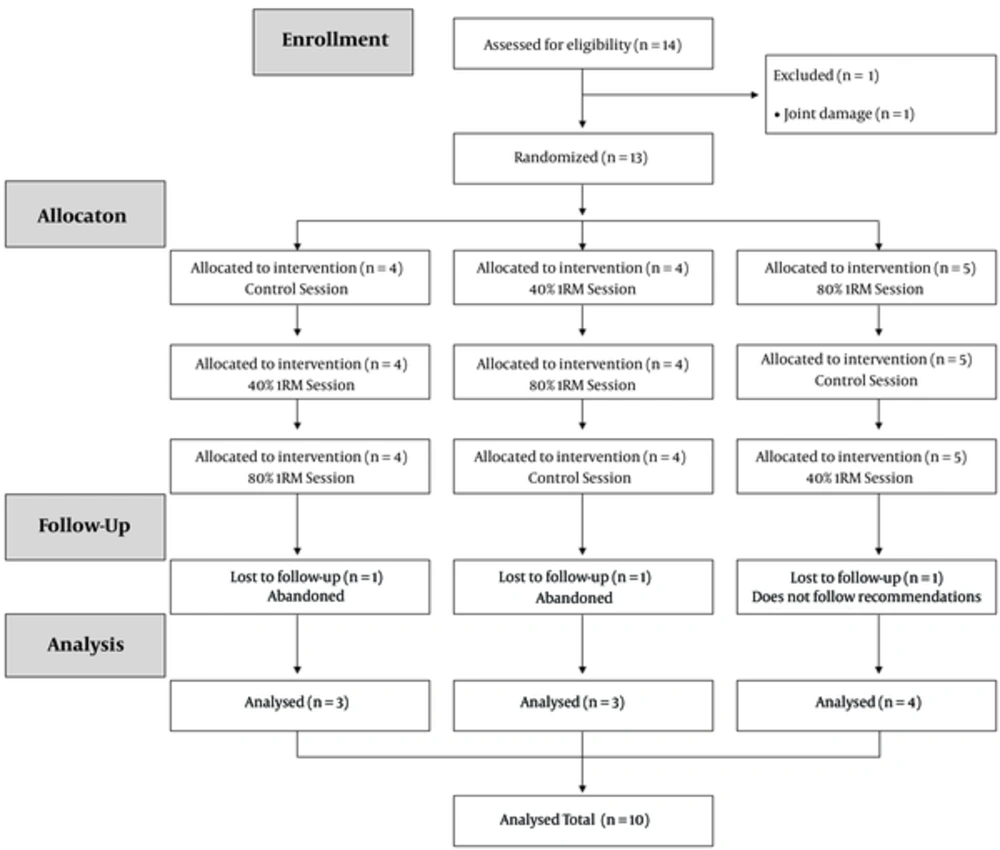
The sample size was calculated statistically using G*Power 3.1.9.2 software (Franz Faul, Universität Kiel, Germany) based on previous studies (8) that observed changes of -3.6 ± 1.7 mmHg in the diastolic BP of healthy subjects after an RE session and 3.4 ± 1.0 mmHg after a control session; thus, considering a power of 80% and an alpha of 5%, the sample size was estimated to be 8 subjects. Estimating a potential sample loss, 14 University professors were invited to participate in the study, however, four were excluded (Figure 1). The participants were informed of the risks, benefits and objectives of the study and signed an informed consent form, which was approved by the research ethics committee of the Universidade Federal do Vale do São Francisco (no. 0002/040613). The research was conducted according to the principles of the declaration of Helsinki. Exclusion criteria were hypertension; cardiovascular disease; obesity; smoking or drug use that could affect the cardiovascular responses during the study; joint, bone or muscle problems that could limit the execution of the experimental sessions; and not complying with the recommendations during the study.
3.2. Experimental Procedure
To test the hypothesis of the present study, after the evaluation of anthropometric characteristics, an interview covering medical history and habits of daily living and a strength assessment using the one repetition maximum (1RM) test, consisting of another three distinct experimental sessions for a period of seven days each and always at the same period of the day and in random order, were applied to University professors. The interventions during each experimental session consisted of RE with intensities of 40% 1RM, 80% 1RM and control without exercise (CONT). Before and 24h after the different interventions, with emphasis on the teaching and sleeping hours of the University professors, the hemodynamic and cardiac autonomic responses were examined.
3.3. General Characteristics and One Repetition Maximum (1RM) Test
On the first visit to the physiology of exercise laboratory, volunteers filled out forms covering medical history, physical fitness level and risk stratification (18). Subsequently, anthropometric measurements of waist circumference, height and body mass were performed with subsequent calculation of body mass index (BMI) according to the procedures described by Lohman et al. (19). Skinfolds were measured to calculate body density (20) and consequent fat percentage (21).
The 1RM test was performed on all participants in the following exercises: bench press, knee extension, seated butterfly, knee flexion, lat pull-down, leg press, and seated row using Evidence® (Cachoerinha, Rio Grande do Sul, Brazil) and Physicus® (Auriflama, Sao Paulo, Brazil) brand equipment. The 1RM test followed the procedures described by Kraemer and Fry (22). The maximum number of four attempts was performed in the same session (23). The physical and functional characteristics of the participants are shown in Table 1.
| Variables | Value |
|---|---|
| No. | 10 |
| Age, y | 33.6 ± 3.3 |
| Years teaching, y | 7.7 ± 1.6 |
| Weight, kg | 82.4 ± 12.4 |
| Height, cm | 177.0 ± 7.5 |
| Body mass index, kg.m2(-1) | 26.2 ± 3.2 |
| Waist circumference, cm | 87.6 ± 7.5 |
| Body fat, % | 14.2 ± 4.2 |
| Resting SBP, mmHg | 120.9 ± 1.9 |
| Resting DBP, mmHg | 75.6 ± 1.2 |
| 1RM Bench press, kg | 67.0 ± 16.0 |
| 1RM Knee extension, kg | 141.7 ± 29.5 |
| 1RM Peck-deck, kg | 61.5 ± 16.4 |
| 1RM Knee flexion, kg | 98.0 ± 23.6 |
| 1RM Let pull-down, kg | 73.7 ± 12.4 |
| 1RM Leg press, kg | 115.8 ± 21.1 |
| 1RM Seated row, kg | 78.6 ± 13.1 |
Abbreviations: DBP, Diastolic Blood Pressure; SBP, Systolic Blood Pressure; 1RM, One Repetition Maximum Test.
3.4. Experimental Sessions
The participants were told to avoid the intake of alcohol and caffeine in the 24h prior to the experimental sessions. In addition, for this same period, the participants were asked to not perform moderate or vigorous physical activity. The experimental sessions were performed in random order (24) and were separated by a period of seven days each, from 9 to 10 am. All sessions were performed on days the professor taught class, which occurred during the University’s night period, between 7 and 10 pm. In the three experimental sessions, the professor taught the same class and the same subject.
Pre-intervention: Before each intervention, the individuals remained seated in the laboratory for a period of 20 minutes in a quiet room without interference from noise and with a room temperature between 22 - 24ºC (11). During this period, every 5 minutes, BP measurements were obtained, and the R-R intervals (the elapsed time between two consecutive R waves between cardiac cycles) were continuously recorded. The mean BP and indicators of heart rate variability based on the R-R interval records were considered for the pre-intervention resting period.
Intervention: The RE intensities were defined based on previous studies (25, 26). These studies found that both low-intensity (40% 1RM) and high-intensity (80% 1RM) RE sessions promoted positive BP responses at periods subsequent to their execution in normotensive individuals. The 40% 1RM and 80% 1RM RE and CONT sessions lasted 40 minutes each. For sessions with RE, three circuits with 7 exercises each were performed in the same sequence of the 1RM test. For 40% 1RM and 80% 1RM, 16 and 8 repetitions were performed for each exercise, with an interval of 60 and 90 seconds between exercises, respectively, and 120 seconds between circuits. The duration of repetition in the exercises, both at 40% 1RM and 80% 1RM, was 3 seconds - 1 second in the concentric phase and 2 seconds in the eccentric phase of the movement. In the CONT session, the participants remained seated in a comfortable chair in the same environment of the RE sessions.
Post-intervention: When the intervention periods were completed, the participants were allowed to bathe for subsequent installation of the equipment for hemodynamic and cardiac autonomic assessment. The BP monitoring was performed in the subsequent 24h, every 15 minutes during the day and every 30 minutes during the night. The R-R interval records were continuously obtained in the subsequent 24h. At the end of the 24h period, the volunteers returned to the laboratory for removal of the measuring equipment. In the ABPM, the tests were considered valid when records occurred with more than 80% validity.
3.5. Hemodynamic and Autonomic Evaluation
All participants were instructed to maintain their daily eating routine on the day of the hemodynamic and autonomic evaluation. A Meditech ABPM-04, properly validated, was used for the hemodynamic assessment of systolic BP (SBP) and diastolic BP (DBP) in the pre- and post-intervention periods (27). A Polar® RS800CX frequency meter (Electo Oy, Kempele, Finland), also with demonstrated validity and reproducibility (28), was used for the cardiac autonomic assessment during the same period. The autonomic indicators examined were 1, linear methods in the time domain from absolute mean records of the R-R interval (RRi); and 2, methods in the frequency domain by spectral analysis through fast Fourier transform, including low-frequency power (0.04/0.15 Hz) in normalized units (LF n.u.) as a marker of sympathetic activity, high-frequency power (0.15/0.40 Hz) in normalized units (HF n.u.) as a marker of vagal activity, and the LF:HF ratio as a marker of the sympathetic-vagal balance. All indicators are described by the European society of cardiology (29), and all analyses were performed using the software Kubios HRV Analysis version 2.0 (Biosignal Laboratory, University of Kuopio, Finland).
All variables (SBP, DBP, RRi, LF, HF and LF:HF ratio) were analyzed pre-intervention and for 24h post-intervention. In addition, the day-period (DP) and night-period (NP) periods were adopted to calculate, from the SBP and DBP, the mean BP during the DP (wakefulness) and NP (sleep) hours. The percentage of nocturnal BP fall (%NBPF) was calculated from the percentage of BP reduction during NP compared to DT (NP:DP ratio) (12). The morning BP surge (MBPS) that corresponds to the morning increase in BP and is calculated according to the difference between morning pressure (the average BP during the 2 hours after awakening) and the lowest NP BP (the average of the lowest BP and the 2 readings immediately preceding and after the lowest value) were also analyzed (30).
3.6. Statistical Analysis
Descriptive statistics with means and standard deviations or standards error of means were adopted. All variables are presented in absolute variations (Δ = the difference between the baseline and various periods after session). Mathematical procedures to calculate the %NBPF and MBPS were performed. Data normality was verified through an exploratory analysis using the Shapiro-Wilk test. Two-way repeated-measures analysis of variance (ANOVA) was applied, reporting “F-ratio” and “P value” to check the main interaction effects of time by session (time × session) and of time (time). When statistically significant effects were found, a post-hoc Tukey test was adopted for multiple comparisons, and the “P” values were reported. Initially, the variable LF:HF ratio had a non-normal distribution; however, the natural logarithmic transformation (Ln) was performed for scale adjustments to meet the normal distribution model (31). The significance level used in the study was P < 0.05, and the software programs used were BioEstat v. 5.3 and Statistica® v. 7.0.
4. Results
The means of valid records obtained in the ABPM were 91.1 ± 2.9% for the CONT session, 91.3 ± 2.5% for the 40% 1RM session, and 93.2 ± 2.2% for the 80% 1RM session.
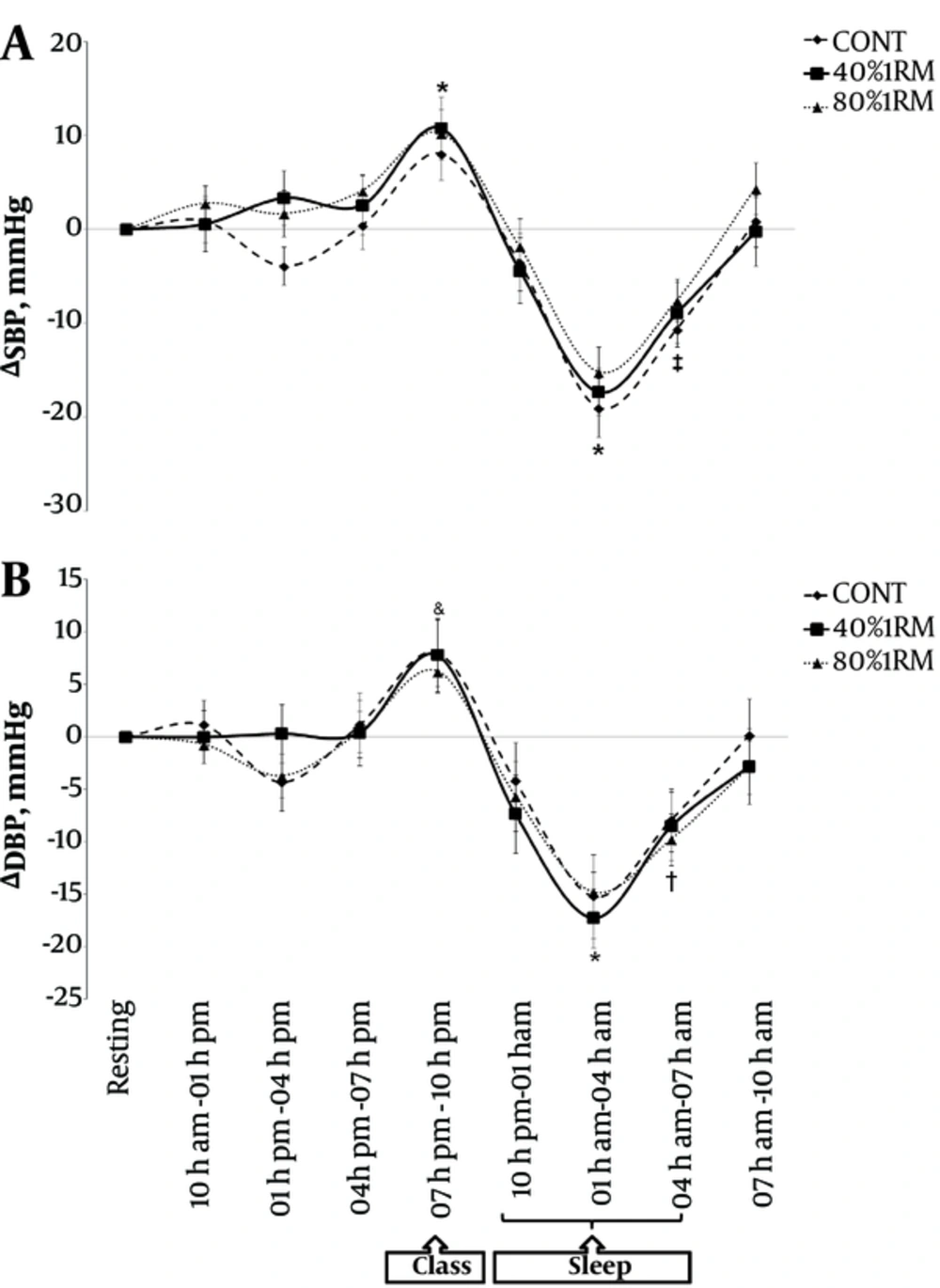
In comparison with the rest values, during the teaching period, SBP increased (Figure 2A) after the three sessions (F(8, 16) = 83.3; P = 0.001) and DBP increased (Figure 2B) only after the CONT session (F(8, 16) = 75.5; P = 0.001). During teaching, there was no difference among the sessions at any period of the 24h for the SBP and DBP responses (F(16, 216) = 0.86; P = 0.615 and F(16, 216) = 0.72; P = 0.772, respectively).
A, Mean (± SEM) of absolute change (Δ) in systolic blood pressure (SBP) and B, Diastolic blood pressure (DBP) during the 24h following experimental sessions (CONT, 40% 1RM, 80% 1RM). * P < 0.05 to CONT, 40% 1RM and 80% 1RM compared with pre-session (Resting); ‡ P < 0.05 to 40% 1RM and CONT compared with pre-session (Resting); & P < 0.05 to CONT compared with (Resting); † P < 0.05 to 40% 1RM and 80% 1RM compared with pre-session (Resting).
Compared with the rest values, during sleep, there were decreases in SBP (Figure 2A) at two periods (1 - 4 a.m. and 4 - 7 a.m.) in the CONT and 40% 1RM sessions (F(8, 16) = 83.3; P = 0.001) and at only one period (1 - 4 a.m.) in the 80% 1RM session (F(8, 16) = 83.3; P = 0.001). In a similar analysis during sleep, there were decreases in DBP (Figure 2B) at two periods (1 - 4 a.m. and 4 - 7 a.m.) in the 40% 1RM and 80% 1RM sessions (F(8, 16) = 75.5; P = 0.001) and at only one period (1 - 4 a.m.) in the CONT session (F(8, 16) = 75.5; P = 0.001). During sleep, there were no differences among the sessions at any period during the 24h period of SBP and DBP responses (F(16, 216) = 0.86; P = 0.615 and F(16, 216) = 0.72; P = 0.772, respectively).
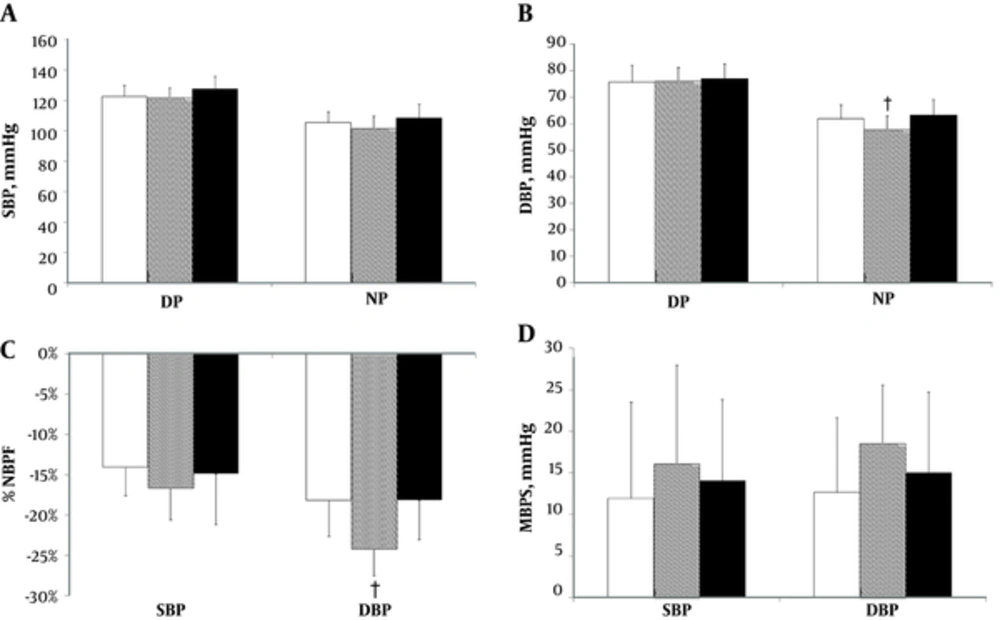
There were no differences in SBP among the three experimental sessions during the DP and NP (F(2, 27) = 1.78, P = 0.187 and F(2, 27) = 1.77, P = 0.189, respectively; Figure 3A). There were significant differences in DBP between the 40% RM session vs. CONT and 80% 1RM sessions in the NP (F(2, 27) = 5.17; P = 0.01; Figure 3B). In contrast, in the DP, the DBP did not differ among the three experimental sessions (F(2, 27) = 0.16; P = 0.852; Figure 3B).
A, Mean (± SD) on day-period (DP) and night-period (NP) of systolic blood pressure (SBP); B, Diastolic blood pressure (DBP); C, Percentage nocturnal blood pressure fall (%NBPF) and D. Morning blood pressure surge (MBPS) in experimental sessions (White bar = CONT, Gray bar = 40% 1RM, Black bar = 80% 1RM). † P < 0.05 to 40% 1RM vs. CONT and 80% 1RM.
In the %NBPF of the SBP variable, no difference was found among the experimental sessions (F(2, 27) = 0.775; P = 0.471; Figure 3C), however, for DBP, there were significant differences between the 40% 1RM session vs. CONT and 80% 1RM sessions (F(2, 27) = 5.75, P = 0.008; Figure 3C). In the MBPS for the SBP and DBP variables, there were no differences among the experimental sessions (F(2, 27) = 0.35; P = 0.707 and F(2, 27) = 1.52; P = 0.236, respectively; Figure 3D).
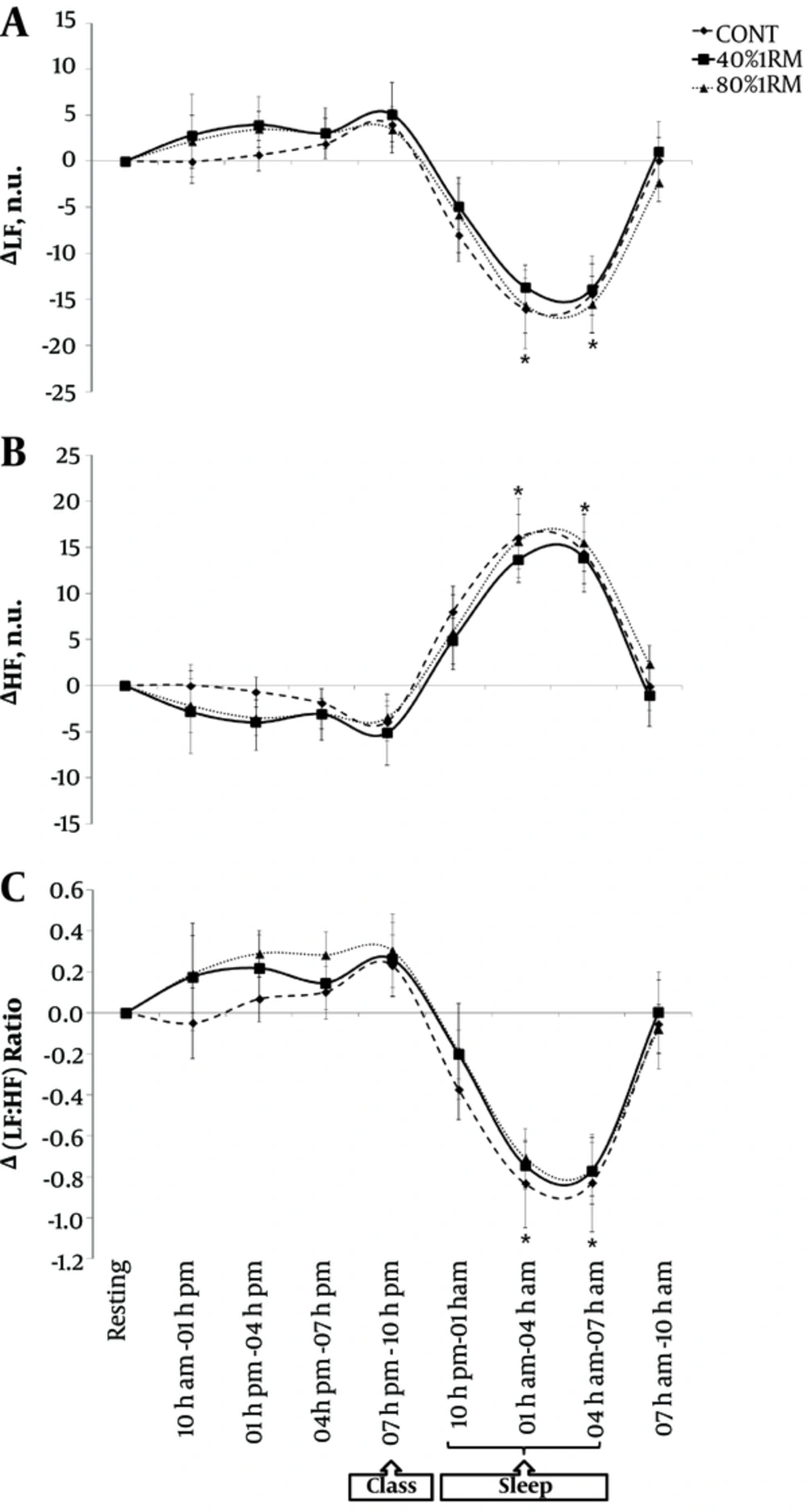
Regarding the rest period, there were significant modulations in the autonomic indicators during sleeping for all experimental sessions. There was a decrease in sympathetic nervous activity, shown in Figure 4A for LF (F(8, 16) = 179.2; P = 0.001) and in Figure 4C for the LF:HF ratio (F(8, 16) = 145.2; P = 0.001), with increased parasympathetic nervous activity for RRi (F(8, 16) = 88.7; P = 0.001) and for HF (Figure 4B) (F(8, 16) = 179.2; P = 0.001). In addition, with the exception of the 80% 1RM session, in the 40% 1RM and CONT sessions, the RRi increased during the 7 - 10 am period of the following day (F(8, 16) = 88.7; P = 0.001).
There were no differences among the experimental sessions at any period during the 24h for the variables RRi (F(16, 216) = 0.93; P = 0.532), LF (F(16, 216) = 0.16; P = 0.999), HF (F(16 216) = 0.16; P = 0.999) and LF:HF ratio (F(16, 216) = 0.21; P = 0.999).
5. Discussion
The main findings indicated that during teaching, the DBP of the University professors did not increase significantly after low- and high-intensity RE sessions (Figure 2B). In addition, during the NP, the DBP showed a smaller absolute value (Figure 3B) and a greater %NBPF (Figure 3C) in the 40% 1RM session. Although there was a higher %NBPF for DBP in the lower intensity RE session, the MBPS did not differ among the experimental sessions (Figure 3D). No differences were observed in any of the sessions compared to rest or among the experimental sessions during the teaching period; however, during sleep, there were significant physiological adjustments in all sessions, though with no difference among them (Figures 4A, B and C). These results suggest that other mechanisms unrelated to the sympathovagal dynamics may explain possible cardiovascular adjustments in University professors after performing RE, especially of lower intensity.
To our knowledge, up to the present, this study is the first to investigate acute cardiovascular responses at different RE intensities in University professors during teaching and sleeping hours. Ribeiro et al. (5) examined the BP responses in University professors after acute sessions of aerobic exercise and found that SBP and DBP were attenuated during teaching compared to the pre-exercise rest period. In the present study, there were positive results in DBP after RE sessions (Figure 2B); however, there was no change in SBP (Figure 2A). The contrasting results between the two studies may be explained due to methodological issues, where the main differences between the studies were the types of exercise adopted (aerobic vs. resistance) and the periods when the professors taught. In Ribeiro et al. (5), the teaching took place in the morning, soon after the completion of the aerobic exercise session, while in the present study, teaching occurred at night, approximately 10 hours after performing the RE sessions.
Monitoring the cardiac autonomic control during work activity can be considered an important and sensitive marker of mental stress alteration at work (3). In the present study, during teaching in the modulations of the autonomic variables, no differences were observed in any of the sessions compared to rest or among the experimental sessions in the University professors (Figures 4A, B and C). In Ribeiro et al. (5), the autonomic indicators could not be examined after exercise; however, some studies (26, 32, 33) demonstrated that after RE sessions of moderate or high intensity, the LF:HF ratio increased. Unlike the present study, previous studies examined the cardiac autonomic tone for only a few minutes after RE. In the present study, when the LF:HF ratio was analyzed for a longer period after RE, no differences were observed in any of the sessions compared to rest or among the experimental sessions (Figure 4C).
During the sleeping hours, the SBP and DBP decreased significantly, with no differences between the RE and CONT sessions (Figure 2A and B). Up to this point, these results corroborate other findings (9, 10, 32, 34). However, unlike those previous studies, when the NP was evaluated, obtained based on the mean DBP during sleep, there was a decrease after the 40% 1RM session compared to the CONT and 80% 1RM sessions (Figure 3B); the same result occurred for the %NBPF (Figure 3C). Flook et al. (12) emphasized that measures that consider BP at sleep in relation to wakefulness (%NBPF) represent indicators with great applicability for the cardiovascular health of an individual. In this sense, these results have important clinical applications for University professors, who execute work tasks during wakefulness that generate high levels of stress (16, 17) and that are associated, after sedentary behavior, with increased BP during teaching and smaller reductions in BP during sleeping (5), with a consequent higher risk of developing systemic hypertension in this population (1).
Moreover, the %NBPF values observed in this study showed 10% - 20% reductions in BP in the three experimental sessions (Figure 3C), which characterizes the sample as dippers (25). However, in the 40% 1RM session, the %NBPF of the DBP exceeded 20%, which classified the sample as extreme dippers (35, 36). These authors (35, 36) noted that the absence of %NBPF is related to higher incidence of encephalic lesions and sleep disturbance, with increases in the sympathetic tone and BP to values similar to wakefulness.
The results of the present study for DBP during sleep (Figures 3B and 3C) and the findings of Ribeiro et al. (5) suggest that regardless of the type of exercise (aerobic or resistance), BP-reducing effects occur during sleep among University professors on the days they teach. Cornelissen et al. (37) showed that RE at lower intensities, similar to that performed in the present study (40% 1RM), should be part of a chronic intervention strategy to prevent and fight high BP. However, more studies are needed to compare the effectiveness of both types of exercise in the magnitude and duration of the hypotensive responses of University professors.
This study also examined the MBPS (30, 38). Cardiovascular events occur more frequently in the morning, and the MBPS is associated with stroke risk regardless of ambulatory BP and decreases in nocturnal BP (38, 39). Despite the %NBPF for DBP being higher for the 40% 1RM session, the MBPS was not different among the three sessions (Figure 3D), and the same was observed for SBP (Figure 3D). This result is in agreement with Rodrigues et al. (9), where no differences were detected between the CONT and 40% 1RM sessions for MBPS in patients with peripheral arterial disease.
Although the objective of this study was not to track the mechanisms associated with BP responses after RE, some authors (26,33) showed that the reduction in BP is associated with decreases in cardiac output and stroke volume that are not offset by an increase in peripheral vascular resistance. Further clarification regarding the mechanistic pathways of reducing or maintaining BP at different periods during the work activity of University professors and the subsequent period of sleep is necessary.
The findings of present study revealed a single session of RE in both intensities (40% 1RM or 80% 1RM) to be effective for acute control of diastolic blood pressure in subsequent teaching hours and only low intensity in subsequent sleeping hours. Thus a low or high intensity RE would be performed in a circuit model to control BP in University professors. The prescription would be done consisting of 3 sets of 7 exercises with 16 or 08 repetitions each at an intensity of either 40 or 80% 1RM, respectively. The recovery between exercises would be between 60 or 90 seconds, respectively, in which the participant should change the exercise, and the muscle groups involved would be the large ones and exercise would be performed alternating them (preferably).
Despite those RE intensities being considered low to high and thus apparently safe for normotensive individuals, in terms of cardiovascular and endocrine stress, a previous medical screening, including an orthopedic, cardiovascular, and metabolic evaluation, is strongly recommended, especially to RE session at higher intensity. In conclusion, this study found that RE at different intensities attenuated DBP during teaching among University professors and that only the 40% 1RM session reduced DBP during sleep in this population. Furthermore, the low RE intensity promoted a greater %NBPF in the sample studied, though without significantly increasing the BP values upon waking.