1. Background
Naproxen and other nonsteroidal anti-inflammatory drugs (NSAIDs) are among the world’s most prescribed medications (1) because, primarily, of their anti-inflammatory and analgesic effects (2). Due to these characteristics and because their use is approved by the World Anti-Doping Agency, NSAIDs are regularly consumed by athletes for ergogenic benefit (3-5).
A study analyzing drug and dietary supplement use by 234 athletes participating in South American games reported that 157 consumed some type of medication immediately before or during competition, and 36.9% were NSAIDs (6). The study also found that NSAIDs were the most commonly used drugs because of their well-established role in treatment of musculoskeletal conditions widespread in athletic competitions (6).
Injuries that affect athletes often result from the need to increase muscular strength, which relates directly to performance improvement in numerous sporting events. To improve muscle strength, athletes must exercise at a level that challenges the neuromuscular system sufficiently to promote physiological and structural adaptations (7, 8). However, such training can also bring about tissue damage and inflammation, resulting in delayed onset muscle soreness (DOMS) and a consequent decrease in muscle strength (9). Despite these detrimental effects, it has been proposed that the acute inflammatory response may be a key element in beneficial post-exercise tissue adaptations (10).
In attempts to gain competitive advantage during strength training, athletes use NSAIDs such as ibuprofen, aspirin, naproxen, and others (6). However, whether consumption of these drugs confers a performance-enhancing benefit remains unclear. One study showed that ibuprofen did not alter the number of repetitions performed in upper or lower limbs, indicating that its use did not alter exercise tolerance during a strength-training session (11). Another study found that ibuprofen had no effect on the histologic appearance of leukocytes in an acute resistance-training (RT) bout (10); furthermore, no effect was found on blood markers of muscle injury or subjective muscle pain. Thus, no current evidence shows that use of ibuprofen contributes to exercise tolerance or influences physiological markers for muscle injury or subjective muscle pain.
2. Objectives
On the other hand, considering that athletes regularly consume NSAIDs for ergogenic benefit and that the literature is scarce on this subject, we investigated whether naproxen enhances neuromuscular performance. We hypothesized that naproxen ingestion would have a beneficial effect on neuromuscular outcomes when consumed prior to a RT session.
3. Methods
3.1. Sample
Participants were a convenience sample of 11 resistance-trained men (5.2 ± 5.0 years’ experience) selected from bodybuilding gyms in the city of Lavras-MG. All participants signed an informed consent (TCLE) approved by the Ethics Committee of the Federal University of Lavras (under CAAE protocol number: 38090314.0.0000.5148) and according to the Declaration of Helsinki.
To be accepted into the study, participants were required to be males from 18 to 30 years of age with at least 1 year of strength-training experience. All prospective participants completed a questionnaire (12), and those who had a chronic medical condition that could create an unnecessary risk during the exercise test or who reported using some type of NSAID were excluded from the study. Participants reported free from use of anabolic steroids were instructed to avoid ingestion of supplements or pharmacological drugs on days of the experiment. Table 1 presents participants’ physical characteristics.
| Variable | Value |
|---|---|
| Age | 24.6 ± 5.5 |
| Hieght, cm | 178.7 ± 5.2 |
| Weight, Kg | 80.3 ± 9.0 |
| BMI, Kg/m2 | 27.63 ± 4.8 |
| Body fat, % | 22.0 ± 3.4 |
| Body fat free, % | 77.9 ± 3.4 |
| Total body water, % | 57.8 ± 2.8 |
| 1 RM, Kg | 98.1 ± 23.1 |
Abbreviations: 1 RM, 1 repetition maximum; BMI, body mass index.
3.2. Experimental Protocol
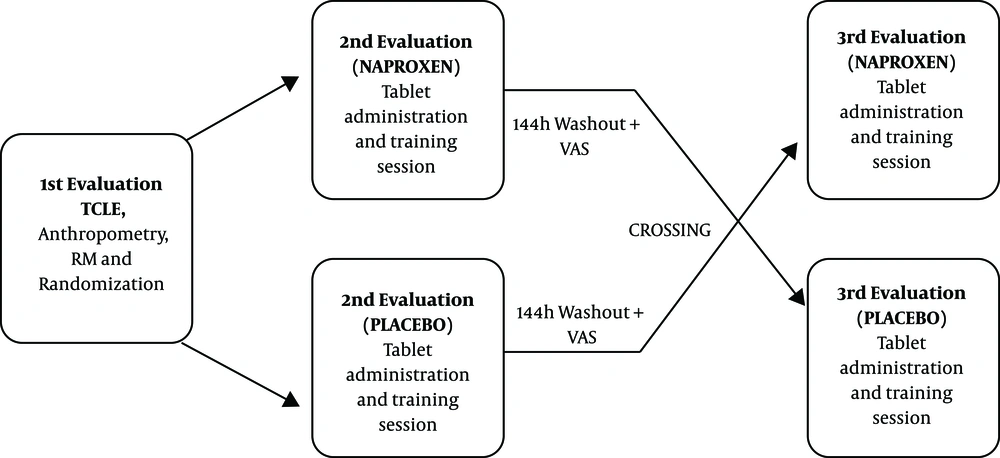
This study employed a crossover, randomized, double-blind, placebo-controlled design, conducted in the laboratory of human movement studies (LEMOH) at the Physical Education Department of the Federal University of Lavras (DEF-UFLA). Participants visited the laboratory on three separate occasions with a 24-hour interval between the first and second sessions and 144 hours between the second and third sessions as per previous studies (13, 14). Randomization was conducted using Microsoft Excel.
The first session included signing the TCLE, randomization of the sample, anthropometric measurements, and 1 repetition maximum (1 RM) testing. Session 2 included strength training and ingestion of a naproxen tablet or placebo. For strength-training sessions, participants consumed a tablet containing either naproxen (tablet 1) or placebo (tablet 2) 1 hour prior to the session. At the beginning of strength-training sessions, participants performed a 30-second warm-up at 30% of 1 RM on the horizontal bench press, followed by a 1-minute rest interval. Subsequently, three maximal sets were performed of the horizontal bench press at 90% of 1 RM, with each set separated by a 2-minute rest interval. Cadence was set at 45 radians per second (2 seconds) for the concentric action and 45 radians per second (2 seconds) for the eccentric action (2/AC for 2/AE) time as controlled by Metronome Plus software. Twenty-four hours after the training session, participants assessed their level of DOMS through the visual analog scale (VAS). Participants reported this measurement daily at the same time each day. DOMS data were recorded via daily telephone contact between participants and research staff. In session 3, participants crossed over, so those who had taken tablet 1 in the first session took tablet 2 in this session and vice versa. Figure 1 provides a schematic of the experimental design.
3.3. Drug Administration
Pharmacological treatment was administered 1 hour prior to each participant’s strength-training session. A 144-hour crossover interval was provided between conditions for administration of naproxen and the placebo. Volunteers ingested a naproxen capsule (500 mg) or a placebo capsule (microcrystalline cellulose) with the same shape, color, weight, odor, and taste as naproxen 500 mg. A single investigator was responsible for randomization and distribution of capsules to participants. Volunteers and researchers had no knowledge of capsules’ contents.
3.4. Anthropometry
Anthropometric measures were performed according to Guedes (15), using the following criteria: (A) not having taken diuretic medication in the last 7 days; (B) having fasted for at least 4 h; (C) not having consumed alcoholic beverages in the last 48 h; (D) not having performed intense physical activity in the last 24 h; (E) urinating at least 30 min before the measurement; and, (F) remaining in absolute rest for at least 8 - 10 min in the supine position before having measurements taken. Height and body mass data were measured in the orthostatic position using a Welmy® scale and stadiometer. The percentage of adipose tissue and fat-free mass was estimated with a Quantum BIA-II® tetrapolar bioimpedance apparatus (RJL Systems, Inc. Clinton: MI, USA) with 3M® electrodes (model 2223BR). For the right foot, the distal electrode was affixed at the base of the middle toe, while the proximal electrode was affixed between the distal epiphyses of the tibia and fibula. For the right hand, the distal electrode was affixed on the base of the middle finger, and the proximal electrode was affixed on the styloid process. All procedures were performed at the same time of day at a controlled temperature of 22 ºC and 75% relative humidity. Data obtained from apparatus’s resistance and reactance were transferred to the software Body Composition 2.1, where the sample’s collected data on height, body mass, and wrist circumference had already been recorded. Participants’ body fat percentage was estimated from these data.
3.5. 1 Repetition Maximum Test
The 1 RM test is characterized by the greatest possible load that a participant can lift for one repetition of an exercise (12, 16, 17). Once participants were acclimated to the equipment and taught the necessary techniques certified by the strength and conditioning specialist, they participated in the 1 RM test. Determination for 1 RM was made for the horizontal bench press as follows: First, participants performed two warm-up sets of two to five repetitions. The load for these sets was established at approximately 50% to 80% of their estimated 1 RM. These warm-ups were followed by sets of increasingly heavier weights with inter-set rest intervals of 5 minutes until a 1 RM weight was established for each participant. The same researcher monitored all 1 RM tests to help ensure good validity (12).
3.6. Strength-Training Session
Exercise intensity and time were adapted from Correa et al. (11). After a 30-second warm-up at 30% of 1 RM in the horizontal bench press exercise, participants from both groups began the test session at 6:00 P.M. Each participant performed three sets of the horizontal bench press exercise at 90% of 1 RM until concentric failure, with a 1-minute inter-set rest interval. Cadence was set at 45 radians per second (2 seconds) for the concentric action and 45 radians per second (2 seconds) for the eccentric action (2/AC for 2/AE) as controlled by Metronome Plus software. Environmental factors such as the noise level (82 dB), temperature (19.0 ± 1.0 ºC), humidity (40% - 50%), and comfort were strictly controlled. Participants were encouraged to achieve as many repetitions as possible in each set until concentric failure.
3.7. Workload
Workload for each set was calculated as the product of repetitions and load. To determine total workload, we summed means of the three sets. The following equations were used for determination of workload:
Repetitions × load set = workload set
Total workload = ∑ workload sets
3.8. Fatigue Index
The fatigue index (FI) was employed to identify the strength loss rate by the equation that Sforzo and Touey proposed (18):
FI = fatigue index and TS = total strength (lifted load × number of repetitions during sets).
3.9. Delayed Onset Muscle Soreness (DOMS)
The visual analog scale (VAS) was used to measure perceived DOMS. The scale is designed to express pain from a straight line with numerical values having a range of 0 to 10, where 0 represents “no pain”, 5 represents “average pain”, and 10 represents “unbearable pain”. The evaluator instructed participants to demarcate the relative value of their perception of pain in the VAS.
3.10. Statistical Analysis
Descriptive statistics (mean ± standard deviation) were used to present all data. Four separate repeated measures analyses of variance were used to compare the number of repetitions, DOMS, workload, and FI for treatment (naproxen versus placebo) and time. With respect to time, the number of repetitions, workload, and FI analysis encompassed the number of sets (n = 3 time points), while for DOMS, the analysis encompassed time points in hours (n = 6 time points). Where indicated, post hoc analysis was conducted through the Bonferonni test for statistically significant effects. Statistical analyses were performed using IBM SPSS statistics software 23 (IBM Corp., Armonk, NY). Results were considered significant at α ≤ 0.05.
4. Results
A significant main effect was observed for time (P < 0.001), with a decrease in the number of repetitions noted across all sets for both treatments (P < 0.05). There was no statistical difference between treatments (P = 0.067) and no interactions between conditions (P = 0.32), although a large relative difference of 44.89% was noted in the third set favoring naproxen (Table 2).
| Group | 1st Set | 2nd Set | 3rd Set |
|---|---|---|---|
| Naproxen | 4.73 ± 2.10 | 4.09 ± 2.07 | 3.55 ± 1.36 |
| Placebo | 4.82 ± 2.22 | 3.73 ± 1.55 | 2.45 ± 1.29 |
| Percentage | -1.86 | +9.65 | +44.89 |
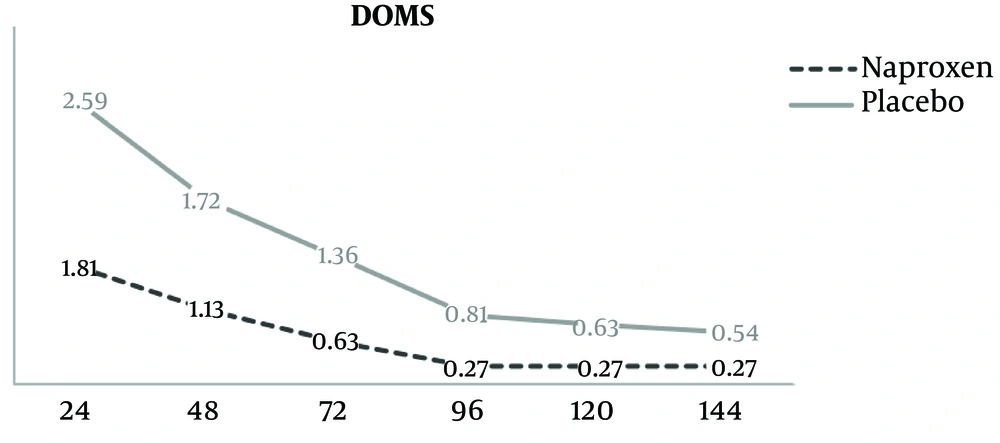
As shown in Figure 2, perceived DOMS was lower in the naproxen group across all time points, but differences were not statistically significant between treatments (P = 0.10). There was a significant main effect for time, with reductions in DOMS noted from 24 to 48h (P = 0.016), 48 h to 96 h (0.033), and 72 h to 120 h (0.017).
Table 3 displays comparison of workload in the three sets between and within groups, and total workload between groups. There was a significant effect for treatment (P = 0.42), with naproxen showing greater total workload across sets compared to placebo. There was also a main effect of time (P = 0.002), with workload significantly decreasing on each successive set, irrespective of treatment (P < 0.05).
| Group | 1st Set | 2nd Set | 3rd Set | Total |
|---|---|---|---|---|
| Naproxen | 410.23 ± 176.43 | 352.47 ± 174.12 | 308.29 ± 127.78 | 1071.00 ± 439.80a |
| Placebo | 409.09 ± 190.63 | 320.07 ± 133.37 | 208.14 ± 127.89 | 937.30 ± 393.60 |
| Percentage | -0.24 | -10 | -32 | -12 |
a Significantly different from placebo.
Table 4 shows comparison of the fatigue index between groups during the three sets. A main effect for time was noted, with a statistically significant difference observed between the second and third sets compared to set 1 (P = 0.003). No main effect for treatment (P = 0.14) or interaction (P = 0.072) was noted.
| Group | FI 1 × 2 | FI 1 × 3 | FI 2 × 3 |
|---|---|---|---|
| Naproxen, % | 13.25 | 18.56 | 5.79a |
| Placebo, % | 4.72 | 52.39 | 29.09 |
a FI 2 × 3 naproxen group × placebo group P (0.02)
5. Discussion
The present study showed that use of naproxen prior to a strength-training session can positively affect performance and alleviate markers of DOMS and muscle fatigue. Findings may be related to the study participants’ high level of physical conditioning. Trained participants require large workloads to generate mechanical overload, which can result in considerable damage to muscle structures (membranes, Z-line, sarcolemma, T-tubules, and myofibrils). According to Grgic et al., higher loads cause greater wear and micro injury and thus necessitate greater recovery (19).
A recent double-blind, placebo-controlled study that provided 1.2 g of ibuprofen showed no statistical benefit of NSAID consumption in the number of repetitions between sets and in total training volume in the bench press and squat exercises at a load of 65% of 1 RM in young men (11). The load used in that study was low; in contrast, the load in our study was high, with potential to provoke a greater inflammatory process and neural fatigue. A recent systematic review found evidence that NSAID ingestion reduces markers of neuromuscular damage after sets performed to concentric failure (20, 21). Moreover, the intake of 1 g of an NSAID also improved quadriceps torque after an intermittent exercise protocol (21, 22). These findings are consistent with those observed in our study, which found that when training with high loads, the number of repetitions, total volume of work, and rate of fatigue improved, indicating that NSAIDs can be ergogenic when consumed prior to strenuous exercise.
Evidence regarding use of NSAIDs to alleviate DOMS remains equivocal, with some studies showing little to no efficacy of ibuprofen (4, 10, 23). For naproxen, Bourgeois et al. showed that a 500-mg dose taken pre- and post-exercise did not decrease perceived DOMS (24). This result is somewhat in contrast to the findings of Brewer et al. (25), who reported that a 440-mg dose of naproxen reduced the response of the metabolite prostaglandin F2α (PGF2α). Prostaglandin F2α (PGF2α) is directly related to the post-exercise inflammatory process, and, consequently, its decreased activation would seemingly lead to lower sensation of DOMS (25, 26). However, given that muscle damage was not measured directly, this finding should be taken with circumspection (25). In the present study, participants reported their perceived DOMS for 1 week, and no significant differences were found in this outcome. Possibly, the use of VAS, an indirect measurement tool, does not necessarily reflect changes in underlying causes of DOMS. In addition, although naproxen has a longer action time than ibuprofen (27), neither has been found to decrease DOMS significantly in most athletes.
One novel aspect of this study is investigation into naproxen’s effects on fatigue indices. Although results did not reach statistical significance, our findings demonstrated that naproxen use resulted in decreased FI, with marked relative differences in the second and third sets between treatments (33.8% and 23.3%, respectively). The literature remains equivocal as to the inflammatory process pursuant to high and low loads; recent studies suggest that training volume, rather than intensity, primarily drives exercise-induced inflammation (28, 29). The naproxen-mediated decrease in FI conceivably occurs via inhibition of synthesis of prostaglandins, endogenous substances produced in the inflammatory process, upon blocking activation of isoenzymes constitutive of cyclooxygenase 1 (COX1) and inductive cyclooxygenase 2 (COX2) (1, 5, 30-32). Large standard deviations noted in this variable indicate that any beneficial effects may be specific to the individual.
The study had several limitations that must be considered when attempting to draw practical conclusions. First, we did not measure metabolic parameters such as lactate, CK, and myoglobin, thus impeding our ability to define potential mechanisms responsible for NSAIDs’ positive effects on acute RT performance. Moreover, our findings cannot be generalized to conclude that all classes of NSAIDs induce an ergogenic effect. Accordingly, future studies should seek to determine potential ergogenic effects of different doses of this class of drugs. In view of these considerations, this study’s conclusions are restricted to the dose of one 500-mg tablet of naproxen taken 1 hour before exercise and to the population studied (young, resistance-trained men).
5.1. Conclusions
The present study demonstrated that ingestion of naproxen had an ergogenic effect on an acute strength-training bout. It should be emphasized that this appears to be the first study to evaluate this drug’s effect on strength-training performance. Novel studies controlling other training variables and providing different doses are necessary for further clarification of naproxen’s effects during exercise.
From a practical standpoint, NSAID use prior to a training session may help increase the number of repetitions and training volume in a RT session. Further experiments should be conducted to verify whether chronic NSAID use continues to enhance performance over time or whether beneficial effects decrease or perhaps become refractory.