1. Background
The low carbohydrate, high fat diet (LCHFD) is a contentious topic and its health-promoting benefits have been questioned (1). This is because traditionally, the consumption of a high-fat diet tends to result in the development of a diverse pattern on dyslipidemia (2). Dyslipidemia as a condition is generally characterized by hypertriglyceridemia, increased low-density lipoprotein cholesterol (LDLC) and decreased high-density lipoprotein cholesterol (HDLC) (3). Dyslipidemia has been found to be a health problem of pandemic proportions that affects both developed and developing countries (2).
Cholesterol is used in the body to aid cell membrane anabolism, synthesis of sex hormones, vitamin D, adrenal gland hormone and secretion of bile that helps with digestion (3). However, before cholesterol enters the bloodstream, it binds to a protein substance, called a lipoprotein (3). Lipoproteins are categorized into high-density lipoprotein (HDLC), low-density lipoprotein cholesterol (LDLC), very low-density lipoprotein cholesterol (VLDLC) and chylomicrons (4, 5). Specifically, an increase in LDLC has been found to be associated with developing atherosclerotic plaque, which contributes to a cellular alteration in the arterial inner walls. Furthermore, this has been found to be more relevant when combined with a decrease in HDL-C, which is responsible for the reverse transport of lipids, especially from the arterial walls (6, 7). Furthermore, HDLC has been found to have an opposite relationship to LDLC. It can counteract the development of cardiovascular disease (CVD) and prevent the occurrence of arteriosclerosis, as it also contributes to the breakdown of the other lipoproteins (7-9). With an increase of LDLC and a decrease in HDLC, it has been noted that the frequency of ischemic heart disease and CVD increases by 2% every time total cholesterol (TC) increases by 1% (10).
Dietary modifications, along with physical activity, are the first line therapy for preventing and treating dyslipidemia (3). This is since diets that are high in fat are generally associated with an elevated TC and LDLC (11, 12). However, limited research has indicated that the opposite may also be true in that an increased dietary fat consumption (especially at the expense of carbohydrates) has been shown to increase HDLC, while simultaneously decreasing LDLC (7, 13). Additional research supporting the role of a high-fat diet at the expense of carbohydrates at improving CVD arises from Muller et al. (14) and Gilmore et al. (15). Specifically, Crouse et al. (16) stated that consuming diets high in polyunsaturated fatty acids might have a lowering effect on TC and improvements in its individual constituents. In addition, unsaturated fatty acids are considered to still be as healthy as a low fat diet, even if the diet has up to 40% unsaturated fatty acids (15, 17, 18).
Along with dietary modifications, physical activity is considered the main intervention for the prevention and treatment of dyslipidemia since diet/caloric restriction alone has been found to not be an effective method of reducing lipoprotein-levels in the long-term. This is because physical activity may result in a decreased protein loss and the maintenance of the metabolic rate along with a concomitant increased fat metabolism (3). Empirical evidence also indicates that physically active individuals have lower TC, triglycerides (TG), and LDLC blood levels, and positively improved HDLC levels, compared to inactive individuals (19-21). Several exercise modalities have been advocated to improve blood lipid profiles with each possibly providing additional specific cardioprotective benefits (22). Recently, more intensive modes of exercise, such as high-intensity interval training (HITT) have been proposed as the most efficient mode of exercise to improve several health measures, including lipoprotein lipids. However, such intensive exercise may not be suitable for sedentary or at-risk populations, such as diabetics. In addition, such intense modes of exercise have been found to induce acute exertional rhabdomyolysis (23). In turn, the benefits of aerobic training in health promotion and positive alterations in lipoprotein-lipids are well documented and this mode of exercise training continues to be the golden standard for exercise and health professionals (24). Furthermore, a lot of scientific literature supports the daily step goals of 10000 steps for adults which equates to approximately walking eight kilometers/five miles and burning 300 - 400 calories (25). However, little/no research has been conducted on the effects of a lifestyle modification intervention that includes a LCHFD, especially in conjunction with physical activity on lipoprotein-lipids. To this point, we hypothesized that an LCHFD alone would not be as effective as an LCHFD combined with physical activity at improving lipoprotein-lipids in a population of type 2 diabetics.
2. Objectives
This study attempted to investigate if a LCHFD, either alone or in combination with physical activity could alter lipoprotein-lipids in individuals with type 2 diabetes.
3. Methods
3.1. Participants
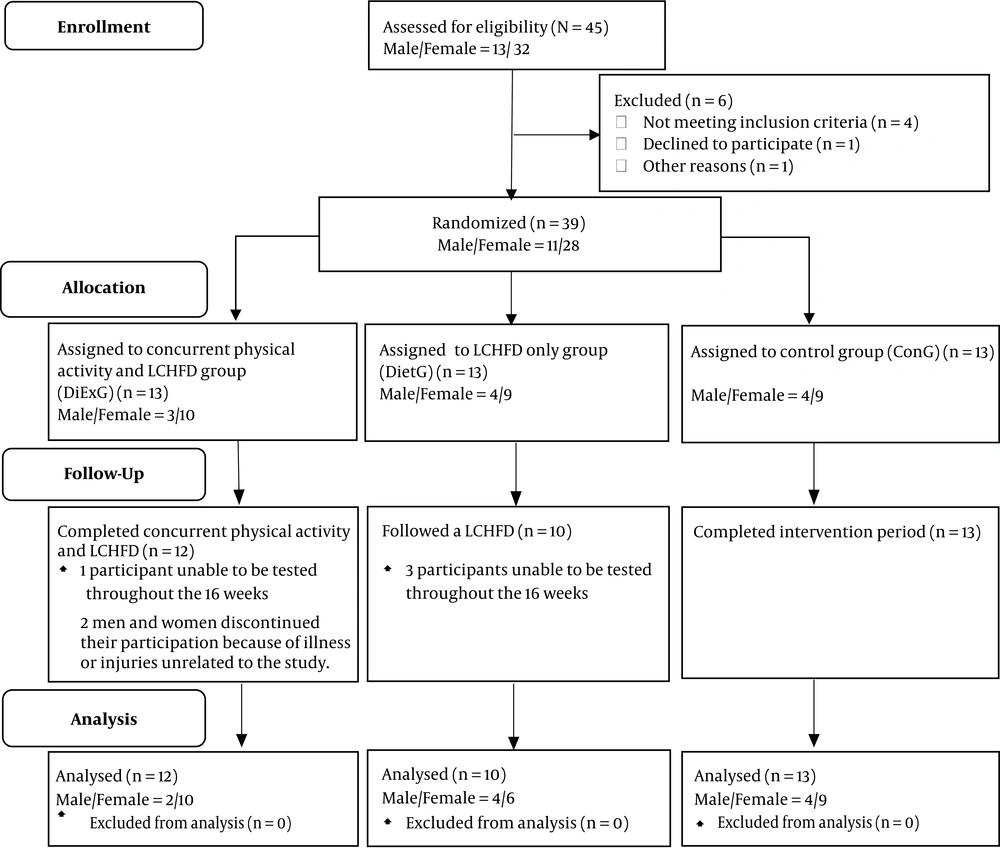
The present study employed a small-scale proof-of-concept investigation using a pretest-posttest design with an intention-to-treat (ITT) analysis (Figure 1). A convenience sample was utilized and participants were recruited from a diabetic clinic in Richards Bay and the surrounding area in South Africa. Participants were screened by the medical doctor at the diabetic clinic prior to participation. Twenty-eight female and 11 male type 2 diabetics aged 31 - 71 years were assigned into a group either participating in simultaneous physical activity and LCHFD (DiExG) (n = 13), or LCHFD alone (DietG) (n = 13) or a blinded control group (ConG) (n = 13). The primary outcome measures were lipoprotein-lipid concentrations in type 2 diabetics following the various interventions. All participants provided written informed consent prior to participation in the study. Eligibility for inclusion in the study was determined using distinct study criteria. It was a requisite that all participants not present with any relative or absolute contraindications to exercise as part of the inclusion criteria (7). Inclusion also required that participants be medically and clinically stable and not beusing ambulant aids. Eligibility criteria required participants to be adults with a diagnosis of type 2 diabetes and no change in their regular medication usage for at least six months prior to the study. Participants were also required to be previously sedentary with no participation in structured/regular exercise for more than twice a week and were required to be weight-stable within approximately two kilograms over the past year.
3.2. Procedures
For descriptive purposes, participants were weighed in kilograms to the nearest tenth of one kilogram on a digital platform scale (Trojan, Model: BSA16056v, Duteck, Taiwan) and stature was determined using a wall-mounted stadiometer to the nearest tenth of one centimeter (Seca Stadiometer, 216, Seca, USA) wearing minimal clothing. Body mass index (BMI) (in kg.m-2) was determined by dividing the measured body mass (in kg) by stature (in m2). Skinfolds (subscapular, tricep, suprailliac, abdominal, thigh and calf) were sampled on the right side of the body using a skinfold caliper (Harpenden, HSB-BI, ATICO Medical Pvt. Ltd, UK). Percentage body fat (%BF) was estimated using the generalized equations of Jackson and Pollock (26).
Blood samples were acquired subsequent to a 9 - 12 hour overnight fast. Venous blood was drawn by a phlebotomist registered with the Health Professions Council of South Africa (HPCSA) and centrifuged serum and plasma were stored at -80°C. Serum TC, LDLC, TG and HDLC were assayed using a Beckman AU 480 apparatus in an accredited pathology laboratory.
3.3. Intervention Program
The DietG and DiExG were required to follow a LCHFD that required participants not to eat more than 50 g of carbohydrates daily (27, 28). Participants in these two groups were provided with three food lists and were instructed that they were able to consume any foods listed on the green list, minimal foods listed on the orange list, but not to consume any foods off of the red list (27, 28). At the first meeting, the primary researcher provided a single set of instructions on how participants were to document food intake to complete the self-report food records. In addition to the LCHFD, the DiExG engaged in 16-weeks of walking a minimum of 10,000 steps daily (29) (measured using a pedometer wristband). The DiExG participants recorded their number of steps daily in a personal physical activity logbook. The blinded control group (ConG) continued their usual activities for the 16-week experimental period.
3.4. Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 25.0 for Windows (SPSS-25) (IBM Corporation, Armonk, NY). Data were reported as means ± standard deviations (SD). For all measured variables in this study, the normality (P > 0.05) of the data were determined by the Shapiro-Wilk test and equal variance (P > 0.05) was determined by Levene’s test. Averages and correlations were calculated using the paired t-test and data were subjected to a one-way analysis of variance (ANOVA). A confidence level of P ≤ 0.05 was considered statistically significant.
4. Results
From the initial 39 participants who were eligible to participate in the study, 35 participants completed the study and were included in the final analysis, of which 12 were in the DiExG, 10 were in the DietG and 13 were in the ConG (Table 1). Four patients were unable to be tested throughout the 16 weeks and were excluded from the final analysis.
| Groups | Group | % | Male | % | Females | % |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| DiExG (n = 12) | 55 ± 9.35 | - | 61 ± 8.49 | - | 53.8 ± 9.45 | - |
| DietG (n = 10) | 54.2 ± 12.67 | - | 58.5 ± 15.02 | - | 51.3 ± 11.36 | - |
| ConG (n = 13) | 58.3 ± 5.53 | - | 62 ± 2.94 | - | 56.7 ± 5.72 | - |
| Gender | ||||||
| DiExG (n = 12) | - | - | 2 | 16.7 | 10 | 83.3 |
| DietG (n = 10) | - | - | 4 | 40 | 6 | 60 |
| ConG (n = 13) | - | - | 4 | 30.8 | 9 | 69.2 |
| Smoking | ||||||
| DiExG (n = 12) | 2 | 16.7 | 1 | 8.3 | 1 | 8.3 |
| DietG (n = 10) | 1 | 10.0 | 1 | 10 | 0 | 0 |
| ConG (n = 13) | 3 | 23.1 | 2 | 15.4 | 1 | 7.7 |
| Body mass, kg | ||||||
| DiExG (n = 12) | 89.4 ± 22.61c | - | - | - | - | - |
| DietG (n = 10) | 104.7 ± 14.16 | - | - | - | - | - |
| ConG (n = 13) | 104.9 ± 32.93 | - | - | - | - | - |
| BMI, kg.m-2 | ||||||
| DiExG (n = 12) | 32.4 ± 7.91c | - | - | - | - | - |
| DietG (n = 10) | 38.9 ± 6.06 | - | - | - | - | - |
| ConG (n = 13) | 38.2 ± 10.66 | - | - | - | - | - |
| %BF, % | ||||||
| DiExG (n = 12) | 37.7 ± 13.75 | - | - | - | - | - |
| DietG (n = 10) | 36.2 ± 15.34 | - | - | - | - | - |
| ConG (n = 13) | 34.8 ± 16.05 | - | - | - | - | - |
Abbreviations: %BF, percentage body fat; BMI, body mass index; kg, kilograms; kg.m-2, kilograms per square meter; y, years.
aValues are expressed mean ± SD.
bDiExG: simultaneous physical activity and LCHFD group; DietG: LCHFD only group; ConG: blinded control group.
cSignificantly (P ≤ 0.05) different (i.e. heterogeneous).
Following the 16-week experimental period, no significant (P > 0.05) changes were found in TC, LDL-C, TG and HDLC across all three groups (Table 2).
| Groups | Pre-Test | Post-Test | P Value | % Difference |
|---|---|---|---|---|
| Total cholesterol, mmol.L-1 | ||||
| DiExG | 4.7 ± 0.93 | 4.8 ± 0.86 | 0.791 | ↑2.0 |
| DietG | 4.9 ± 1.71 | 4.9 ± 1.54 | 0.881 | 0.0 |
| ConG | 5.2 ± 1.38 | 5.3 ± 1.36 | 0.981 | ↑1.9 |
| Triglycerides, mmol.L-1 | ||||
| DiExG | 2.1 ± 1.83 | 1.9 ± 1.32c | 0.477 | ↓9.5 |
| DietG | 2.3 ± 1.59 | 2.8 ± 3.51 | 0.677 | ↑17.9 |
| ConG | 3.8 ± 4.93d | 3.3 ± 2.24 | 0.836 | ↓13.1 |
| LDL-cholesterol, mmol.L-1 | ||||
| DiExG | 2.5 ± 0.49 | 2.7 ± 0.86 | 0.704 | ↑7.4 |
| DietG | 2.9 ± 1.25 | 2.5 ± 1.20 | 0.744 | ↓13.8 |
| ConG | 2.7 ± 1.08 | 2.8 ± 1.50 | 0.940 | ↑3.6 |
| HDL-cholesterol, mmol.L-1 | ||||
| DiExG | 1.3 ± 0.29 | 1.3 ± 0.29 | 0.989 | 0.0 |
| DietG | 1.1 ± 0.28 | 1.1 ± 0.25 | 0.844 | 0.0 |
| ConG | 1.2 ± 0.35 | 1.2 ± 0.28 | 0.998 | 0.0 |
Abbreviations: ConG, control group; HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol; mmol·L-1: millimoles per liter; TC, total cholesterol; TG, triglycerides.
aValues are expressed mean ± SD.
bDiExG: simultaneous physical activity and LCHFD group; DietG: LCHFD only group.
cSignificantly (P ≤ 0.05) different at post-test (i.e. heterogeneous).
dSignificantly (P ≤ 0.05) different at baseline (i.e. heterogeneous).
5. Discussion
The primary intention of this study was to determine if a LCHFD provides any benefits on lipoprotein-lipids, either alone or in conjunction with physical activity in type 2 diabetics. The major result of present study is that a 16-week LCHFD with or without physical activity does not have any benefit on lipoprotein-lipids in type 2 diabetics. Specifically, no significant changes were observed in TC, TG, LDLC and HDLC in either the DiExG or DietG.
Following the 16-week experimental period, TC was found to be relatively stable in all three groups. This study’s findings are in agreement with Thompson et al. (13) who previously demonstrated that a high-fat diet failed to elicit any improvements in TC. This finding and that of Thomson et al. (13) are in contradiction to the findings of Crouse et al. (14) who purport the substitution of SFA with polyunsaturated fatty acids might have a lowering effect on TC. In terms of low-carbohydrate diets, Volek et al. (30) demonstrated that very low carbohydrate diets (VLCD) (< 50 g carbohydrates), as utilized in the present study, actually resulted in a harmful increase in TC. In terms of exercise, previous studies have demonstrated that exercise has little/no effect on TC levels (21). This may not be due to the inability of exercise to alter any specific biological mechanism, but rather that TC is a generalized measure of lipoprotein-lipids and includes the “good” (i.e. HDLC) and “bad” (i.e. LDLC) components of cholesterol and any increase and/or decrease in its constituents creates an unchanged TC.
While it has been claimed that TG is the most consistent and predictable of the lipid changes when a LCHFD is used (31), in this study, TG increased, albeit non-significantly, by 17.9% in the diet-only group and supports the general assumption that TG is only reduced using a low-fat diet (32). More disconcerting is the finding that physical activity failed to improve TG in this study. This may indicate that the addition of the high-fat diet actually inhibited the TG-lowering benefits of exercise. This is because exercise, irrespective of modality, is associated with decreased serum levels of TG (33, 34). Further, evidence for the interference effect of a high-fat diet on physical activity is supported by Lian et al. (35) who have found that even walking, such as utilized in this study, can lower TG.
This study’s findings are in agreement with Thompson et al. (13) who previously demonstrated that a high-fat diet failed to elicit any improvements in LDLC. In terms of low-carbohydrate diets, Volek et al. (30) demonstrated that very low carbohydrate diets (VLCD) (< 50 g carbohydrates), as utilized in this study, actually resulted in a harmful increase in LDLC. Again, this study uniquely demonstrates that the use of a LCHFD may deleteriously counterpoise the positive effects of physical activity in that exercise, even walking, may decrease LDLC levels (33, 34).
High-density lipoprotein is considered cardioprotective and every 0.03 mmol.l-1 increase in total HDLC translates into as much as a 2% to 3% reduction in CVD risk (36). While previous research has indicated that diets that substitute fats with carbohydrates are associated with a lowered HDLC (7), the present study indicated no change in HDLC following the 16-week experimental period. In addition, Volek et al. (37) found that the consumption of a low-carbohydrate diet increases HDLC, especially when compared to a low-fat diet. Once again, even the concurrent group that also engaged in exercise failed to elicit any improvements in this parameter. This is problematic in that exercise, even walking as utilized in this study, is associated with an improved HDLC (33-35). One possible reason for a lack of improvement in the DiExG may be due this study’s 16 weeks being of insufficient intensity or duration not meeting the time of latency for this mode of exercise to improve HDLC (21).
The lack of change in the measured lipoprotein-lipid profiles in this study are difficult to attribute to any specific mechanism. In this regard, the specific biological mechanism(s) responsible for adaptations in lipoprotein-lipids following dietary and/or exercise interventions are as yet unknown (24). It is for this reason and due to the limited amount of scientific research on the influence of LCHFDs on lipoprotein-lipid profiles, especially in individuals with diabetes, that the health benefits of LCHFDs remain controversial.
5.1. Limitations
The present study had some limitations. Due to the small sample, results should not be universally directed to the entire diabetic populace. In addition, the study did not utilize a single gender and differences in gender hormones and their effect on lipoprotein-lipids could have affected the results. While there are challenges with using dietary self-report measures and pedometry as a tool for physical activity measurement, both will continue to be popular approaches due to the lack of inexpensive and more sensitive objective means of assessment. Further, it is unclear whether a longer intervention period would result in positive improvements since the effect of exercise may require a certain time of latency (such as required on HDLC) before the changes can be proved.
5.2. Conclusions
In conclusion, we found that 16-weeks of a low carbohydrate, high fat diet, alone or in conjunction with physical activity, did not improve lipoprotein-lipids in type 2 diabetics and may actually result in unfavorable, albeit insignificant, lipoprotein-lipid adaptations. In fact, it appears that health professions should not unquestionably include LCHFDs in a diabetic treatment regime to manage or improve lipoprotein-lipid in type 2 diabetics. Rather health professionals should apply proven well-established dietary guidelines on an individual needs basis.