1. Background
Drug therapy is essential for the management of type 2 diabetes mellitus (T2DM). Nevertheless, physical exercise should be focused on from the beginning of diabetes care as part of the lifestyle management (1). Exercise exerts effects beyond physical fitness in these patients, such as controlling the plasma glucose levels and lowering oxidative stress; therefore, exercise program development should be encouraged as part of the nonpharmacological side of diabetes management in order to ensure proper β-cell functions (2, 3). Given the wide range of exercises, training (4, 5), and lifestyle modifications (6) that are available for T2DM management, experts agree that designing a training program is challenging for a number of reasons. For example, the heterogeneity of diabetic patients should be considered (7), both the dose and effects must be measured, and the program should be adjusted to each individual’s condition (8) and physical ability. Any complications, comorbidities, and contraindications should also be taken into account, as well as the individual’s preferences (8-10). The program must be sufficiently dynamic in order to hold the patient’s interest, while also being safe, and it must increase the patient’s participation through the prescription of clear exercises to reach realistic goals. The training dose refers to the type, intensity, duration, and frequency of exercise (8), and it should be increased gradually (11, 12). From the perspective of T2DM patients, it is only natural that the training facility should be easy to access, and the schedule should accommodate the patient’s daily routine.
Structured cardiorespiratory and/or resistance exercises are known to be effective for T2DM management (1, 13) and recent studies have looked at interval training as an alternative form of cardio exercise for diabetes management (14). One significant issue for exercise-based T2DM management that includes interval and resistance training (15-17) is the potential for excessive reactive oxygen species (ROS) levels, leading to oxidative stress-related tissue damage, especially when it causes exhaustion (18). Therefore, oxidative stress is an important marker because a high plasma glucose level will also increase the production of ROS (19). Fortunately, exercise can still play a useful role in T2DM management when the intensity is controlled to yield low ROS levels (20).
2. Objectives
For this study, the primary endpoints were a glycated hemoglobin A (HbA1c) level reduction and increased physical fitness, while controlling the oxidative stress level, through 12 weeks of a combined high-intensity interval training (HIIT) and resistance training (RT) program. All of the other confounders, including medication, diet, and physical activity, were unchanged throughout the trial in order to ensure minimal outside effects on the exercise program.
3. Methods
This randomized controlled trial (RCT) was scheduled for 12 weeks. For this research, an HIIT and RT combination program for T2DM exercise management was designed with a gradual increase in the exercise intensity (18). The study was granted approval by the Ethical Committee for Health Research, Faculty of Medicine, at the Universitas Indonesia, Dr. Cipto Mangunkusumo Hospital (protocol number 17-05-0554).
3.1. Interventions
The patients in the experimental (EXP) group participated in a combination of HIIT and RT, as shown in Table 1.
| Week | HIIT | RT | |||
|---|---|---|---|---|---|
| Intensity | Duration | Set | Rep | Load/RPE | |
| 1 | 60% - 70% HRmax | 20 minutes | 1 | 8 | No external weight-minimal weight/3 - 4 |
| 2 | |||||
| 3 | HIE: 90% HRmax; LIE: 70% HRmax | 20 minutes HIIT consists of HIE: 4 one minute cycles (total of 4 minutes); and LIE (recovery): 4 four minutes cycles after each HIT (total of 16 minutes) | 1 | 10 | External weight resistance/5 - 6 |
| 4 | |||||
| 5 | |||||
| 6 | |||||
| 7 | HIE: 92% HRmax; LIE: 75% HRmax | 30 minutes HIIT consists of HIE: 6 one minute cycles (total of 6 minutes); and LIE (recovery): 6 four minutes cycles after each HIT (total of 24 minutes) | 2 | 10 | External weight resistance/7 - 8 |
| 8 | |||||
| 9 | 2 | 10 | External weight resistance/8 - 9 | ||
| 10 | |||||
| 11 | |||||
| 12 | |||||
Abbreviations: HIE, high intensity exercise; HIIT, high intensity interval training; HRmax, maximum heart rate; LIE, low intensity exercise; Rep, repetition; RPE, rating of perceived exertion; RT, resistance training.
Each HIIT element was comprised of cycles of one minute of high-intensity exercise followed by a recovery period consisting of four minutes of low-intensity exercise (21). The intensity was determined by the maximum heart rate (HRmax), which was estimated from the maximum oxygen uptake (VO2max) using the Swain regression equation (22). The training program began with a low-moderate exercise volume designed to ease the patient into the exercise session. The heart rate data were gathered using a Polar H7 heart rate monitor (Polar Electro Inc., Bethpage, NY, USA), which was linked to an iPad application. For the HIIT exercise, the subject could choose either a treadmill or an ergocycle.
The RT was comprised of nine exercises using free weight resistance or weight machine resistance to train the muscles in the whole body (23). The resistance was gradually increased using the rating of perceived exertion as the lead-in to support the development of both muscle strength and endurance (24). The first two weeks were designed as an adaptation period to build muscle memory for each movement. From the third week on, leg extension, leg curl, dual adjustable pulley, and lat. exercise machines were used.
The control (CTR) group performed continuous cardiorespiratory-only exercises once a week, starting with the same exercise volume as the experimental group. After 2 weeks, the intensity was gradually increased. The exercise load for weeks 3 - 6 was set at 70% - 75% of the HRmax for 25 minutes. For the remaining 6 weeks, it was set at 75% - 80% of the HRmax for 30 minutes. All of the exercise sessions for both groups began and ended with 10 stretching movements. Each cardio session included five-minute warm-up and cool-down periods on the exercise machine.
The exercise programs were conducted at the Center for Sports and Exercise Studies at the Indonesian Medical Education and Research Institute at the Universitas Indonesia Faculty of Medicine (IMERI FKUI). The treadmills, ergocycles, and weight machines (Technogym USA Corp., Fairfield, NJ, USA) were available, and a sports medicine specialist was on duty to supervise each exercise. A subject did not begin exercising if his or her resting blood pressure and heart rate exceeded 180/100 mmHg and 100 beats per minute, respectively, or if his or her blood glucose level fell outside the 100 - 300 mg/dL range. Any health problems caused by the exercise activities were recorded and attended to.
At the beginning of the study, all of the subjects received information about the training program, and they each provided consent. The subjects were told not to change their lifestyles (6, 21), and a monthly consultation with the primary investigator was conducted to monitor each subject’s medications and health issues (10). The step averages were measured using pedometers (HJ325; OMRON, Kyoto, Japan), energy outputs using Bouchard’s three-day physical activity records, and energy inputs using three-day food intake records were analyzed using the NutriSurvey program (Southeast Asian Ministers of Education Organization Tropical Medicine and Public Health Network Regional Center for Community Nutrition - University of Indonesia), and they were recorded every four weeks. As agreed upon beforehand, a mobile phone message reminder was sent to each subject one day before each exercise session.
3.2. Participants
All of the T2DM patients, aged 18 - 64 years old, who were registered at primary clinics near the training facility and who were regularly taking oral hypoglycemic agents for at least 6 months were recruited for this research. The inclusion criterion was a controlled plasma glucose level predetermined by the upper limit of the average fasting plasma glucose level of 250 mg/dL or equal to an HbA1c level of 10.5% (25). Those subjects with diabetic complications, such as macroangiopathy, severe retinopathy, severe nephropathy, or restricted locomotion, were excluded from this study. Finally, the subjects were randomly allocated to either the EXP or CTR group using a block of four randomization.
3.3. Outcome Measures
For each patient, the HbA1c, the fitness level, malondialdehyde (MDA), and superoxide dismutase (SOD) blood values were measured before and at the end of the program. The VO2max, as part of the physical fitness determination, was measured using Ebbeling’s submaximal aerobic fitness test from the Canadian Society for Exercise Physiology protocol (26). One-sided blinding was used for this study, which meant that those individuals measuring the fitness levels were unaware of the subjects’ group allocations. In addition, all of the blood samples were numbered using the recruitment order to conceal the group allocations. The fitness levels were measured at the Center for Sports and Exercise Studies at the IMERI FKUI. The HbA1c levels were measured at the Clinical Pathology Laboratory at Dr. Cipto Mangunkusumo Hospital, and the MDA and SOD levels were measured at the Biochemistry and Molecular Biology Laboratory at the Universitas Indonesia Faculty of Medicine.
3.4. Statistical Analysis
The statistical analysis was performed using IBM SPSS Statistics for Windows, Version 20 (IBM Corp., Armonk, NY, USA). The group numerical data were reported as means ± standard deviations, while the non-normally distributed data were reported as medians and interquartile ranges (IQRs). At the outset, the randomization of the EXP and CTR groups was tested using an Independent-samples t-test for the mean differences or a chi-squared test for the differences in the proportions. The between-group effects were analyzed in terms of the post-study and pre-post difference (Δ/delta) measurements using an Independent-samples t-test for the normally distributed data and an independent samples Mann-Whitney U test for the non-normally distributed data. At the end, a composite variable of the main outcomes was created and analyzed. The significance level was set at P < 0.05.
4. Results
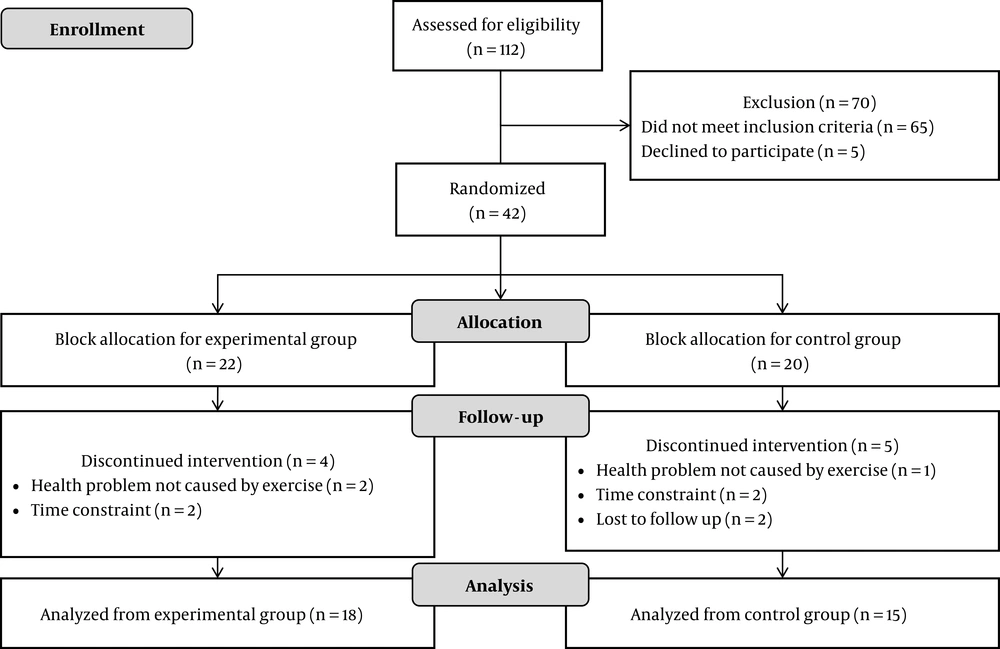
This RCT followed the process illustrated in Figure 1. Of the 112 candidates, 42 patients aged 35 - 64 years old (14 males and 28 females) were deemed eligible as subjects. They were randomized into an EXP group (22 subjects) and CTR group (20 subjects).
The between-group analysis confirmed that, at the baseline, the randomization was successful, as shown in Table 2.
Abbreviations: BMI, body mass index; CTR, control group; EXP, experimental group; N, number of subjects.
aValues are expressed as mean ± SD, unless it was mentioned.
bIndependent-samples t-test;
cPearson’s chi-squared test
At the end of the study, nine subjects had dropped out (Figure 1), therefore the analysis of the results was based on 33 subjects. As shown in Table 3, the main effects of the training program were measured in terms of the glycemic control (HbA1c), fitness level (VO2max), and oxidative stress (MDA concentration and SOD activity). During the study, four out of the 814 sessions were problematic due to health conditions unrelated to diabetes. All of these were resolved, and all four subjects resumed the program.
| EXP (N = 18) | CTR (N = 15) | P Value | |||||
|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | p PRE | p POST | p Δ | |
| HbA1c (%) | 7.96 ± 1.11 | 7.53 ± 0.73 | 8.02 ± 1.67 | 7.70 ± 1.32 | 0.915b | 0.656b | 0.831b |
| VO2max (mL/kg/min) | 25.27 ± 7.80 | 38.13 ± 5.93 | 22.77 ± 4.98 | 32.09 ± 5.24 | 0.293b | 0.004b* | 0.106b |
| MDA concentration (nm/mL) | 0.99 ± 0.26 | 0.85 ± 0.19 | 0.81 ± 0.29 | 0.99 ± 0.22 | 0.068b | 0.055b | 0.011b* |
| SOD activity (U/mL) | 0.40 (0.34 - 0.50) | 0.81 (0.53 - 1.17) | 0.46 ± 0.17 | 0.60 ± 0.32 | 0.464c | 0.062c | 0.036c* |
Abbreviations: CTR, control group; EXP, experimental group; HbA1c, glycated hemoglobin A; MDA, malondialdehyde; N, number of subjects; PRE, initial result; POST, post-training result; p POST, between group difference (EXP or CTR) post-training; p PRE, between group difference (EXP or CTR) at beginning of training; p Δ, change difference between EXP and CTR groups; SOD, superoxide dismutase; VO2max, maximum oxygen uptake.
aValues are expressed as mean ± SD or median (IQR).
bIndependent-samples t-test.
cIndependent-samples Mann-Whitney U test.
At the end of the 12-week training program (Table 3), despite the lower HbA1c level in the EXP group (7.53 ± 0.73%), there was no significant difference when compared to the CTR group (7.70 ± 1.32%, P = 0.656). There was no significant difference in the analysis of the HbA1c changes between the groups either.
However, the VO2max in the EXP group showed a significant increase. In addition, there were significant differences between the EXP and CTR groups in the MDA concentration change (-0.14 ± 0.39 nm/ml and 0.18 ± 0.26 nm/ml, respectively, P = 0.011) and the SOD activity change [0.47 U/mL (IQR 0.08 - 0.74 U/mL) in the EXP group and 0.14 ± 0.35 U/mL in the CTR group (P = 0.036)].
| Category/Score | Var1 (VO2max) | Var2 (HbA1c), % | Var3 (Δ MDA), nm/mL | Var4 (Δ SOD), U/mL |
|---|---|---|---|---|
| 1 | Poor | > 9 | ≥ 0.16 | < 0.23 |
| 2 | Fair & good | 7.01 - 9 | -0.31 - 0.159 | 0.23 - 0.909 |
| 3 | Very good & excellent | ≤ 7 | -0.31 > | ≥ 0.91 |
Abbreviations: HbA1c, glycated hemoglobin A; MDA, malondialdehyde; SOD, superoxide dismutase; Var, Variable; VO2max, maximum oxygen uptake; Δ, change between pre and post-training.
aCut off points for VO2max referred to Canadian Society of Exercise Physiology standard (26); HbA1c categories referred to glycemic control targets stated by the consensus for type 2 diabetes mellitus management in Indonesia (27); MDA and SOD cut off points followed three equal divide of pre- and post-training values (Δ) taken from measurement in this study.
A composite variable of the main outcomes was created to analyze the comprehensive training effects of the main outcomes. The first step in creating the composite variable was to recode all of the continuous variables into three categories of new variables (Var1 - Var4), as shown in Table 4. Then, the sum of the scores was calculated for each variable. The value of the new composite variable for the EXP group (8.72 ± 1.27) was significantly higher than that for the CTR group (7.20 ± 1.08, P = 0.001).
Using a one-way repeated measures analysis of variance, all of the physical activity and food intake analyses showed no significant differences between the measurements from the first, second, and third months, as well as those based on the allocation.
5. Discussion
This study implemented combined HIIT and RT exercises to provide a novel and dynamic form of cardiorespiratory exercise with due attention paid to musculoskeletal issues. It fulfilled the need to obtain evidence from interval and resistance training programs in order to increase the prospects of implementation in clinical settings for various populations with diverse conditions (10, 28-31). However, the study limitations relate to the small size of the cohort (n = 42), with n = 33 at the end of study. The heterogeneous nature of the group was also realized and addressed with a random allocation and statistical analysis to ensure that the outer effects were addressed properly.
The combined HIIT and RT as an alternative exercise program presented itself as a reasonable training program that might also improve diabetes management (14, 30, 32). However, the metabolic profile of T2DM patients makes them vulnerable to high intensity exercises. A graded increase in the intensity and close supervision (33, 34) were required to avoid the risk of a metabolic emergency (14, 16).
After a complete evaluation of the composite training effects, and given that the physical activity and food intake were ruled out as potential confounding factors, the evidence suggests that this combined HIIT and RT program was confirmed as a sound exercise program for T2DM management. The small difference in the HbA1c values found here and in other HIIT/combined HIIT and RT studies (16, 30, 35) may reflect the influence of other factors, such as the average HbA1c formation time (36, 37) or oral medication (30). Nonetheless, there were HIIT studies reported reduced plasma glucose after each exercise session (7) as well as significant glycemic control effects (6, 15, 21, 38). Different effects between studies could relate to difference in detailed exercise design, subjects’ age group, as well as the heterogenous of T2DM clinical conditions. However, studies regarding the effect of combined HIIT and RT were not conclusive to determine its effectiveness on glycemic control (21, 30).
The greatest strength of the training program was the significant lowering of the oxidative stress, as measured using the MDA and SOD values. The graded increase in both the HIIT and RT loads yielded a steady level of reactive species at low concentrations (20, 23, 39). In agreement with one previous study using the same protocol (21), a relatively long recovery duration provided sufficient time to cover the post-high intensity metabolic and respiratory oxygen demands (7, 33). This prevented the accumulation of anaerobic by-products and effectively avoided the unaccustomed and/or exhaustive exercise that can generate excessive ROS, leading to oxidative stress-related tissue damage and impaired muscle contractility (18). The findings also confirm the fact that combined HIIT and RT can increase the antioxidant defenses through the increased production of endogenous antioxidant enzymes, such as SOD, glutathione peroxidase, and catalase (18, 39).
Combined HIIT and RT in T2DM also improve cardiovascular and respiratory function, which are strongly associated with improvements in vascular health (34). The increased VO2max found in this study aligned with those from many other T2DM cardio and interval training studies (15, 21), which may have been influenced by movement efficiency obtained from using a treadmill for both the training and measurement. Effect of combined HIIT and RT on vascular health presented as improved peripheral arterial stiffness indices and distensibility coefficients in a one-year randomized controlled trial (34). These long-term effects suggested that planned periodization of training program should be considered in exercise programming in T2DM management (30, 34).
Protective measures used to guarantee the subject’s health when performing an exercise program are important, and several issues must be considered when implementing this training program. The low percentage of eligible patients supports the evidence for a comprehensive screening of the subject’s health and exercise risks, suggesting that pre-exercise examinations are important factors in T2DM exercise management (30). Although there was only a low number of them, problematic exercise sessions demonstrate the importance of using safety measures during the exercise sessions, such as supervision by trained medical personnel and the personalizing of the training load in an ecological exercise setting (33, 34).
5.1. Conclusions
The structured, combined HIIT and RT exercise program in this study was not significantly improving glycemic control. However, it lowered oxidative stress and the overall composite effects of improved glycemic control, lowered oxidative stress, and increased physical fitness level showed its positive effect.