1. Background
Obesity is one of the most important health problems associated with metabolic and cardiovascular diseases (1). Chronic inflammatory status in obesity leads to endothelial dysfunction, the first step of atherosclerosis leading to cardiovascular diseases. This chronic inflammation is due to increase in inflammatory and reduction in anti-inflammatory cytokines involved in atherosclerosis (2). In obesity, TNFα is considered as an inflammatory cytokine with an increasing innate property, while IL10 is considered as an anti-inflammatory cytokine with a decreasing innate property (3). PTX3 is a member of the Pentraxin superfamily, produced by fibroblasts, monocellular phagocytes, and endothelial cells (4). In animal studies, PTX3 is known as an anti-inflammatory protein and has atherosclerotic and cardiovascular protective effects (4). However, it has been demonstrated that the expression of PTX3 is decreased in adipose tissues and skeletal muscles in obese rodents compared to a control group (5).
Physical activity can modulate the systemic inflammation induced by obesity through elevated anti-inflammatory cytokines (6-8). Moderate-intensity exercises decreased TNFα in sedentary individuals (9), and endurance trained men had higher IL10 compared to sedentary individuals (10). Continuous aerobic training increased IL10 in obese women with metabolic syndrome (11). Also, participating in regular continuous moderate exercise sessions could increase PTX3 in middle-aged women (12). What is more, endurance trained men had higher levels of plasma PTX3 compared to sedentary individuals (13), and acute submaximal aerobic exercise increased PTX3 in healthy obese individuals (14, 15). In all of these studies, moderate continuous body weight bearing exercises were practiced. HIIT is considered as an enjoyable and time-efficient alternative to moderate continuous exercise in controlling the inflammatory response in obesity (3), while most obese individuals do not attend HIIT sessions because of inadequate muscle strength, foot and ankle pain, lower-back pain and shortness of breath (16).
Stationary ergometer can eliminate the above-mentioned problems. However, for increasing active muscle mass in exercise, it may be appropriate to train both the upper and lower body. To the best of our knowledge, there is a scarcity of studies on the effect of this type of exercise on anti-inflammatory cytokines in overweight and obese individuals.
2. Objectives
Thus, we aimed to evaluate the effect of all ex. HIIT on anti-inflammatory PTX3, IL10 and inflammatory TNFα in overweight and obese women.
3. Methods
3.1. Subjects
Thirty female students aged 18 - 23 years old participated in this study. The Ethics Committee of the University of Isfahan (Iran) approved the study protocol (code: 1396.041), and informed consent was obtained from each participant prior to the study. The inclusion criteria consisted of healthy, sedentary, overweight and obese women with body mass index (BMI) ≥ 27 kg/m2. The exclusion criteria included diabetes, cardiovascular diseases, hormonal complications, use of drugs or any kind of supplements, dietary restriction, physical activity (> 2 D/week), smoking and pregnancy. The subjects were equally assigned to training and control groups. The training group participated in all ex. HIIT, while the control group continued sedentary lifestyle with no changes in dietary habits or daily physical activity during the study period.
3.2. Study Design
This study was extracted from a dissertation with a cross-sectional, semi-experimental design. The subjects were randomly assigned to all ex. HIIT and control groups. Randomization was based on coin tossing. Subjects in both groups were instructed to maintain and follow their daily physical activity and diet. The independent variable was all ex. HIIT and the depended variables were anthropometric and blood analyses consisting of PTX3, IL10, TNFα, triglyceride (TG), cholesterol (CHOL), high-density lipoprotein (HDL), low-density lipoprotein (LDL), resting heart rate (HR) and resting blood pressure (BP), which were measured at baseline and 48 hours after 10 weeks of the intervention in a fasting state (Table 1).
| Variable | Age | Height | Weight | BMI |
|---|---|---|---|---|
| All ex. HIIT | 20.20 ± 1.30 | 162.80 ± 5.00 | 78.60 ± 13.30 | 38.80 ± 2.20 |
| Control | 20.70 ± 1.70 | 160.60 ± 5.50 | 82.80 ± 14.30 | 39.90 ± 2.70 |
Anthropometric Characteristic of All ex. HIIT and Control Groups
3.3. Testing Evaluation
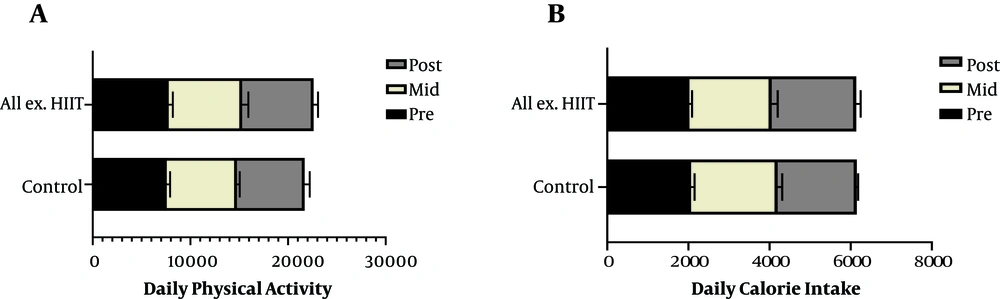
After 12 hours of fasting, the anthropometric variables of the subjects consisting of height and weight were measured by a scale (SEGA, model 220, Germany) with minimum clothing. BMI was calculated by the W (kg)/H2 (m) equation. After 20 min of resting in the sitting position, a blood pressure monitor (Omron MX3, England) and pulse meter (Polar F4, Finland) were applied to evaluate the resting BP and HR. Daily physical activities (step/day), excluding the training program, were assessed by a pedometer (iHealth Edge, Inc. USA) at pre, mid and posttest. These activities were measured three times, two weekdays and one weekend. The obtained means thereof were compared within and between the groups. Daily calorie intake was assessed by the NUT 4 questionnaire, and its diet analyzer software was modified to fit the Iranian food table (17) at pre, mid and posttest on two week days and one weekend each, and the mean of the time series was compared within and between the groups.
3.4. Blood Analysis
A volume of 10 cc fasting blood sample was first taken and centrifuged at 4000 rpm for 20 min, and then it was stored at -80ºC. The levels of PTX3 (MyBioSorce kit, Cat. # MBS825009, USA, sensitivity < 10 pg/mL, detection rate 312 - 20000 pg/mL) (18), TNFα (Bio legend ELISA MAX Deluxe Set kit, USA, Cat.#430204, sensitivity 2 pg/mL, detection rate 7.8 - 500 pg/mL) (19), and IL10 (Bio legend, ELISA MAX Deluxe Set kit, USA, Cat.#430604 , sensitivity 2 pg/mL, detection rate 3.9 - 250 pg/mL) (20) were measured by applying the enzyme-linked immunosorbent assay (ELISA) according to the manufacturers’ instructions. TG, CHOL, HDL, LDL FBS and insulin were measured by the photometric method (Auto analyzer alpha classic, Iran). HOMA-IR was calculated using the following equation:
HOMA-IR = FBS (mg/dL) × Insulin (mU/L)
3.5. Exercise Training
Upper and lower ergometers (Monark 839 and 831, Sweden) were applied for training in a simultaneous manner. Pulse meter was applied to monitor HR during training. The maximum HR was calculated by 220-age. All ex. HIIT included a 10-min warm up phase at 60% - 70% HR max intensity followed by a 4 × 4 min-exercise at 85% - 90% HR max intensity and a 3 × 3 min-active recovery at 60% - 70% HR max in between (21). The last active recovery was for 5 min as the cool down phase.
The sessions were held four times a week (40 min/day) for 10 weeks. The first week was considered as pre-conditioning and familiarization. According to gradual increase in exercise intensity principle, the training intensity was considered 65% - 70% HR max or RPE 12-13 in the first week, 70% - 75% HR max or RPE 13-14 in the second, 75% - 80% HR max or RPE 14-15 in the third and 85% - 90% HR max or RPE 16-17 in the forth to the tenth weeks (22). During this period, the members of the control group did not change their daily physical activity and calorie intake as checked through a pedometer and diet analyzer software (NUT-4) modified to fit the Iranian food table (Figure 1).
3.6. Statistical Analysis
All the statistical analyses were performed in SPSS (version 22; SPSS Inc., Chicago, IL, USA). Shapiro-Wilk test and Q-Q plots were applied for checking the normality of data distribution. Levene’s test was performed to assess the homogeneity of variances. Paired samples t-test and Wilcoxon were run for the evaluation of parametric and nonparametric data, respectively. T-test and analysis of covariance (ANCOVA) were applied for comparisons within the groups, and pretest scores were considered as covariates. Repeated measures ANOVA and Krouskal-Wallis were applied to compare daily calorie intake and daily physical activities, respectively. Variables were recorded as the mean± SD for normal and as the median (interquartile range) for non-normal data. Level of significance was set at 0.05. Pearson and spearman correlations were applied to assess the correlation between PTX3 and other variables in parametric and non-parametric data, respectively.
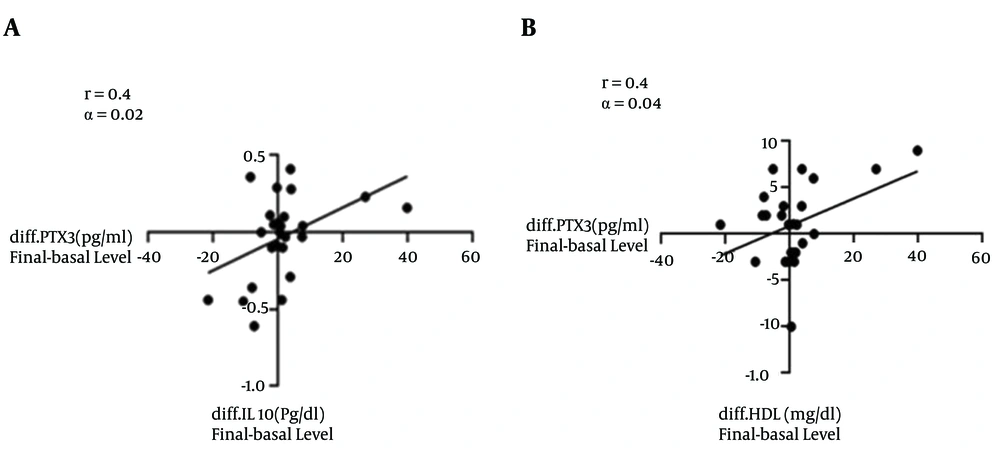
Responses of circulating PTX3, IL10, TNFα, glycemic and lipid profile to all ex. HIIT: No significant changes were observed in PTX3, IL10, TNFα, TG, CHOL, HDL, FBS, insulin and HOMA-IR in the groups. LDL decreased by 13.5% in the all ex. HIIT group compared to its baseline at (P = 0.02) and at (P < 0.001) between groups (Table 2). PTX3 had no significant correlation with TG, CHOL and LDL, while it was positively correlated with IL10 (r = 0.43, P = 0.02) and HDL (r = 0.4, P = 0.04; Figure 2).
| Variable | Groupa | Pb | Pc | |
|---|---|---|---|---|
| Pre | Post | |||
| PTX3 (ng/mL) | ||||
| All ex. HIIT | 25.83 ± 17.63 | 26.98 ± 21.08 | 0.77 | 0.47 |
| Control | 35.39 ± 22.74 | 31.25 ± 19.57 | 0.10 | |
| TNFα (pg/mL) | ||||
| All ex. HIIT | 13.24 (36.520) | 9.30 (13.97) | 0.31d | 0.92 |
| Control | 18.36 (12.63) | 9.30 (12.41) | 0.10d | |
| IL10 (pg/mL) | ||||
| All ex. HIIT | 1.46 ± 0.23 | 1.52 ± 0.32 | 0.46 | 0.67 |
| Control | 1.53 ± 0.29 | 1.52 ± 0.32 | 0.94 | |
| TG (mg/dL) | ||||
| All ex. HIIT | 120.73 ± 37.89 | 130.61 ± 45.47 | 0.22 | 0.54 |
| Control | 107.62 ± 44.57 | 113.46 ± 40.51 | 0.17 | |
| CHOL (mg/dL) | ||||
| All ex. HIIT | 155.61 ± 19.82 | 147.76 ± 21.18 | 0.18 | 0.06 |
| Control | 165.10 ± 26.43 | 166.15 ± 24.66 | 0.40 | |
| HDL (mg/dL) | ||||
| All ex. HIIT | 43.69 ± 10 | 45 ± 11.19 | 0.35 | 0.8 |
| Control | 44.38 ± 7.67 | 45.24 ± 7.54 | 0.38 | |
| LDL (mg/dL) | ||||
| All ex. HIIT | 88.48 ± 23.92 | 76.56 ± 22.62 | 0.02 | ≤ 0.001 |
| Control | 100.03 ± 23.32 | 97.36 ± 26.88 | 0.19 | |
| FBS (mg/dL) | ||||
| All ex. HIIT | 81.07 ± 7.31 | 82.92 ± 6.03 | 0.26 | 0.34e |
| Control | 76.84 ± 8.73 | 76.57 ± 8.89 | 0.35 | |
| Insulin (mU/L) | ||||
| All ex. HIIT | 17.24 ± 9.23 | 14.63 ± 7.27 | 0.21 | 0.56 |
| Control | 14.66 ± 4.88 | 14.58 ± 7.88 | 0.88 | |
| HOMA-IR | ||||
| All ex. HIIT | 3.07 ± 1.72 | 3.53 ± 1.96 | 0.31 | 0.56 |
| Control | 2.82 ± 1.50 | 2.84 ± 1.39 | 0.82 | |
Effect of All ex. HIIT on Anti-Inflammatory Factors, Lipid and Glycemic Profiles
Response of cardiorespiratory function to all ex. HIIT: resting HR declined by 12% in relation to its baseline at (P < 0.001) and at (P < 0.001) between the groups. Systolic and diastolic BP decreased by 2.3% and 6% in the all ex. HIIT group, respectively, while they remained unchanged in the control group (Table 3).
| Variable | Group | Pa | Pb | |
|---|---|---|---|---|
| Pre | Post | |||
| BP systole (mmHg) | ||||
| All ex. HIIT | 111.53 ± 7.09 | 101.99 ± 8.88 | 0.01 | 0.01 |
| Control | 109.84 ± 6.49 | 107.53 ± 7.11 | 0.27 | |
| BP diastole (mmHg) | ||||
| All ex. HIIT | 68.76 ± 5.50 | 64.69 ± 5.54 | 0.06 | 0.02* |
| Control | 66.76 ± 8.36 | 65.46 ± 7.96 | 0.24 | |
| RHR (beat/min) | ||||
| All ex. HIIT | 83.92 ± 10.15 | 73.69 ± 10.45 | < 0.001 | < 0.001 |
| Control | 86.61 ± 10.94 | 86.53 ± 12.61 | 0.90 | |
Effect of All Extremity HIIT on Cardiovascular Factors
4. Results and Discussion
In this study, the effects of all ex. HIIT on PTX3, IL10, TNFα, lipid and glycemic profiles, resting HR and BP of overweight and obese women were assessed. A significant reduction in LDL, resting HR and BP of subjects following all ex. HIIT was observed with no changes in PTX3, IL10, TNFα, TG, CHOL and HDL. Here, all ex. HIIT caused no changes in circulating PTX3. To the best of our knowledge, this is the first study where the effect of all ex. HIIT on PTX3 has been assessed in overweight and obese women. In most of the available studies, the acute effect of exercise on PTX3 was assessed and an elevated concentration in plasma were reported (23, 24). According to (25), 12 weeks of modification in diet and aerobic training decreased weight and increased PTX3 in elderly and obese individuals. The same researchers reported that walking and jogging were used as weight-bearing activity.
Here, non-weight-bearing exercises with all extremities was assessed where a modest weight reduction was observed, while PTX3 remained unchanged. The observed results might be due to the nature of non-weight bearing exercise or its duration.
IL10 is an anti-inflammatory cytokine which is impaired in obesity (26). Here, IL10 remained unchanged after all ex. HIIT. Our findings were consistent with those of (27, 28) as to changes in IL10 after short-term and long-term lower ergometer HIIT, respectively. According to (29), IL10 was elevated in active healthy elderly men. Thus, it could be deduced that longer duration of training may affect the level this cytokine. Although both PTX3 and IL10 as athero-protective molecules showed no significant changes, differences in pre-post measurements between these two variables showed significant correlations. It is suggested that PTX3 as a component of innate immunity is regulated by IL10 (4). In this study, in a 10-week period, TNFα remained unchanged after the intervention, which corresponds to the findings in (27), where eight weeks of aerobic training (cycling) caused no changes in TNFα in obese individuals (27). Conversely, eight weeks of aerobic training and dietary restriction reduced TNFα in overweight adolescents in (28). Despite the hypothesis that all ex. HIIT decreases TNFα, it appears that long duration of exercise or weight-bearing training might reduce this inflammatory marker.
At the end of the intervention, all glucose and lipid profile markers remained unchanged, except for LDL, which decreased in the training group. There is some conflicting data regarding the effect of HIIT on lipid profile and glucose in obese adults. According to (28, 30), weight-bearing HIIT led to no changes in TG, CHOL and HDL. Researchers in (18, 29) demonstrated that all ex. HIIT could not change FBS in obese individuals, while others reported that glucose and lipid profile improved after HIIT (30). It is reported that improvement in lipid profile after exercise may be related to the baseline level (29).
In this study, LDL was deceased, indicating an important health benefit. Based on the findings of (31), any 1% decrease in LDL may reduce the risk of cardiovascular diseases (CVD) by 2%. Despite the lack of change in HDL, it was found to be positively related to PTX3. Therefore, changes in these two variables are related to each other and with longer period of training, better results could be observed in this area. In this study, reduction in resting HR and systolic and diastolic BP was observed. HR and BP are powerful predictors of total cardiovascular mortality (32). Decreased vascular resistance and catecholamine level are related to the reduction of HR and BP after exercise (33). As to the reduction in HR and BP through HIIT, our findings correspond to those of (31, 32), while they are contradictory with the results of (34). The differences in these results may be due to the type and duration of the HIIT and the general conditions of the subjects.
Strengths and limitations of the study: This was the first study run on the effect of all ex. HIIT among overweight and obese women and the whole period was supervised by an exercise physiologist with HR monitoring. This study also had some limitations such as: (1) including only young female subjects, which limits the generalizability of the outcomes to male or older subjects and (2) being confined to a 10-week period, otherwise, better results would have been obtained.
4.1. Conclusions
It was found that non-weight bearing all ex. exercise is appropriate for overweight and obese individuals having problems with weight bearing exercises. All ex. HIIT after 10 weeks could not significantly change inflammatory markers including PTX3, IL10 and TNFα in the training group, while the changes in PTX3 were related to changes in IL10 and HDL. All ex. HIIT decreased resting HR, BP and LDL, hence decreasing the risk of CVD in overweight and obese women. Further studies are needed to clarify the beneficial effects of all ex. HIIT in overweight and obese individuals.