1. Background
Labor induction is a usual obstetric practice that occurs in 20% of pregnancies (1-3). Successful induction minimizes the time to vaginal delivery and improves maternal and neonatal outcomes. Oxytocin is frequently used as a safe and effective initiator of uterine contractions; however, the success of labor induction in this method depends on the favorable or unfavorable cervix at the initiation of induction (4).
An unfavorable cervix, as demonstrated by low Bishop scores (< 4) (5), has an impact on the duration of induction, the likelihood of vaginal delivery, and the cesarean delivery rate (6). The cervical ripening process has been effectively demonstrated to reduce the duration of induction and cesarean section rate and increase the successful vaginal delivery rate compared to induction with oxytocin in women with an unfavorable cervix (1, 6).
The two primary techniques for cervical ripening are mechanical and pharmacologic intervention. Mechanical methods include the insertion of balloon catheters through the endocervical canal, and pharmacologic intervention includes the administration of prostaglandin E1 (misoprostol) and prostaglandin E2 analog (dinoprostone) (4, 7).
Prostaglandins are currently the preferred cervical ripening method (2, 8, 9). Widespread use of dinoprostone has been limited due to high financial costs and challenging storage requirements compared to misoprostol (10).
Furthermore, in a previous large-scale meta-analysis, misoprostol was associated with lower cesarean delivery rates compared to placebo, oxytocin, or dinoprostone (11). Although still controversial, vaginally administered misoprostol may be more effective in time to delivery compared to oral misoprostol, with lower rates of gastrointestinal adverse effects but higher rates of tachysystole (12).
Oral administration of misoprostol has also been associated with more meconium-stained amniotic fluid and aspiration, possibly due to higher peak plasma concentration and fetal exposure (13). While a lower dosing regimen may reduce the risk of tachysystole and uterine rupture, lower doses of oral misoprostol (50 vs. 100 mcg) have been previously demonstrated to have reduced efficacy (14).
As such, reaching a consensus regarding the optimal dosage for the vaginal route would be beneficial in improving maternal and neonatal outcomes in pregnant women requiring cervical ripening and labor induction, especially in a Bishop score of the cervix of 2 or less.
2. Objectives
This trial aimed to compare the efficacy and adverse effects of 50 mcg vs. 25 mcg of vaginal misoprostol in low-risk term pregnant women with a Bishop score of the cervix of 2 or less.
3. Methods
This double-blind, single-center, randomized clinical trial included 200 low-risk term pregnancies with a Bishop score of the cervix of 2 or less undergoing cervical ripening and augmentation from June to August 2022. Pregnant women with a singleton pregnancy, gestational age ≥ 39 weeks (based on the first-trimester ultrasound), amniotic fluid index > 5, Bishop Score of the cervix < 2, reactive non-stress test (NST), cephalic presentation fetus, and no evidence of uterine contractions were included in this study.
Exclusion criteria were pregnant women with premature rupture of membrane, history of uterine surgery or uterine anomaly, chorioamnionitis, allergy to prostaglandins, diseases such as asthma, gestational/overt diabetes mellitus, seizure, preeclampsia, and fever, placenta previa, and any anomaly or intrauterine growth retardation in their fetuses.
At first, the pregnant woman underwent vaginal examination to assess her Bishop score and NST. If the Bishop score was ≤ 2 and NST was reactive without effective uterine contraction (< 3 contractions in half an hour), she was enrolled in the study.
The study indications of pregnancy termination were spontaneous labor pain, the fetus movement decrease, bleeding, and rupture of membrane. Using the reported values (15), a study power of 80%, a two-sided significance level of 5%, and the sample size formula for proportion studies, we calculated the sample size of 100 in each group as follows:
The corresponding author randomly assigned the participants into A (25 µg vaginal misoprostol) and B (50 µg vaginal misoprostol) groups (each containing 100 subjects) using the four-block method.
In this double-blinded study, the participants and the analyst did not know the type of treatment. Because of receiving similar drugs, the participants did not know their treatment types. Also, the analyst did not know the treatment group codes in the SPSS data sheet.
Groups A and B received 25 mcg and 50 mcg vaginal misoprostol, respectively, every six hours up to four times or if the active phase of labor was reached. During the trial, fetal and uterine contractions were monitored. Furthermore, the progression of vaginal examination was assessed every 2 - 3 hours in the latent phase and every 1 - 2 hours in the active phase of labor. If the Bishop score reached more than 4 without sufficient uterine contractions, augmentation with oxytocin was performed. Amniotomy was performed if no increase in the Bishop score was achieved even after medical induction.
The study information included maternal age, BMI, gravidity, history of abortion, baseline Bishop score, and gestational age. The primary outcomes were the interval from misoprostol administration to the end of the first phase of labor (6 cm dilatation of the cervix), the second phase of labor (the time interval from 6 cm to full dilation of the cervix), and the third phase of labor (the interval from full dilatation of the cervix to birth). Other outcomes included the numbers of prescribed misoprostol, the need for additional oxytocin for augmentation, the rate of cesarean section, Apgar scores, umbilical artery pH, and neonatal intensive care unit (NICU) admission.
All data were analyzed using SPSS version 24.0. A P-value of less than 0.05 was determined as the level of statistical significance. The mean ± standard deviation for continuous variables and frequency and percentage for qualitative variables were used.
4. Results
There was no statistically significant difference between the two study groups regarding maternal age, BMI, gravidity, history of abortion, baseline Bishop score, and gestational age (Table 1). The mean time from misoprostol administration to the end of the first phase of labor (6 cm dilatation of the cervix) (P-value = 0.002), the second phase of labor (the time interval from 6 cm to full dilation of the cervix) (P-value = 0.030), and third phase of labor (the interval from full dilatation of the cervix to birth) (P-value = 0.020) were significantly less in 50 micrograms of misoprostol than in 25 micrograms of misoprostol (Table 2).
| Variables | Vaginal Misoprostol | P-Value | |
|---|---|---|---|
| 25 μg | 50 μg | ||
| Age | 25.6 ± 4.5 | 25.1 ± 3.9 | 0.680 |
| Body mass index | 30.1 ± 3.8 | 30.5 ± 3.9 | 0.520 |
| Gravidity | 1.7 ± 0.8 | 1.6 ± 0.9 | 0.300 |
| Parity | 1.4 ± 0.6 | 1.5 ± 0.6 | 0.290 |
| No. of abortion | 1.0 ± 0 | 1.1 ± 0.38 | 0.770 |
| No. of children | 1.3 ± 0.48 | 1.4 ± 0.49 | 0.100 |
| Bishop score | 1.06 ± 0.87 | 1.07 ± 0.89 | 0.800 |
| Gestational age | 40.21 ± 1.32 | 40.35 ± 1.63 | 0.100 |
| Outcomes | Vaginal Misoprostol | P-Value | |
|---|---|---|---|
| 25 μg | 50 μg | ||
| First phase of labor, hours | 1.63 (2 - 57) | 1.23 (1 - 52) | 0.002 |
| Second phase of labor, hours | 3.94 (1 - 10) | 3.13 (0.5 - 9) | 0.030 |
| Third phase of labor, minutes | 5.18 (5 - 120) | 3.77 (7 - 120) | 0.020 |
a Values are expressed as mean (minimum - maximum).
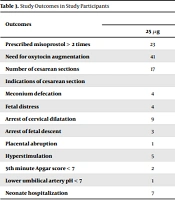
There were no significant differences regarding the numbers of prescribed misoprostol (P-value = 0.300), the need for additional oxytocin augmentation (P-value = 0.100), and cesarean section rate (P-value = 0.500) between the two study groups. Furthermore, the groups were statistically similar in uterine tachysystole, 5-minute Apgar scores < 7, umbilical artery pH < 7, and NICU admission (Table 3).
| Outcomes | Vaginal Misoprostol | P-Value | |
|---|---|---|---|
| 25 μg | 50 μg | ||
| Prescribed misoprostol > 2 times | 23 | 12 | 0.300 |
| Need for oxytocin augmentation | 41 | 32 | 0.100 |
| Number of cesarean sections | 17 | 21 | 0.500 |
| Indications of cesarean section | 0.400 | ||
| Meconium defecation | 4 | 5 | |
| Fetal distress | 4 | 4 | |
| Arrest of cervical dilatation | 9 | 6 | |
| Arrest of fetal descent | 3 | 0 | |
| Placental abruption | 1 | 2 | |
| Hyperstimulation | 5 | 10 | 0.100 |
| 5th minute Apgar score < 7 | 2 | 4 | 0.400 |
| Lower umbilical artery pH < 7 | 1 | 5 | 0.900 |
| Neonate hospitalization | 7 | 7 | 1.000 |
5. Discussion
Vaginal misoprostol has been demonstrated to be superior in efficacy over the oral route, with similar or higher doses, and the oral-plus-vaginal route (8, 16). However, it is still unclear whether pregnancies requiring cervical ripening and labor induction would benefit from higher doses of vaginal misoprostol without compromising maternal or neonatal health.
The present study demonstrated the better effectiveness of 50 mcg vaginal misoprostol in time to delivery compared to 25 mcg vaginal misoprostol in term pregnancies with ≤ 2 Bishop Scores prior to induction. Despite a trend toward a lower need for oxytocin augmentation, the results did not reach statistical significance. Furthermore, there was no significant difference between the study groups in the cesarean section rate or the cause leading to cesarean. These findings align with the results of the meta-analysis by Hofmeyr et al., in which higher doses of misoprostol did not reduce cesarean sections and the need for oxytocin augmentation (4).
Overall, this study supports the safety and efficacy of the higher dose of misoprostol in low Bishop scores of the cervix with no significant increase in adverse maternal or neonatal outcomes. Nevertheless, there was a trend toward higher rates of tachysystole in those receiving 50 mcg of misoprostol. Guidelines have also suggested an increased risk of tachysystole with 50 mcg of misoprostol compared to 25 mcg dose (17), with meta-analyses demonstrating an increased risk of uterine tachysystole with non-reassuring NST with higher doses of misoprostol in women with the unfavorable cervix (4). Although this study noted inclusion and exclusion criteria of enrolled women and used misoprostol in ≤ 2 Bishop score of the cervix instead of ≤ 4 in the other survey, we did not experience any difference in uterine tachysystole between the two groups.
At least half of the women undergoing labor induction may require cervical ripening due to the unfavorable cervix (1). Thus, using agents to ripen the cervix has become part of regular care in clinical obstetrics. Successful cervical ripening, labor induction, and augmentation methods aim to reduce the time from induction to delivery with minimal maternal and neonatal adverse effects. A considerable proportion of failed labor inductions and unnecessary cesarean sections could be prevented by proper choice of the ripening agents and their optimal dosage for cervical ripening (14, 18, 19).
Despite the growing evidence, misoprostol has not yet been approved by the Food and Drug Administration (FDA) for cervical ripening prior to labor induction (20, 21). The findings of this study contribute to the existing literature on the use and safety of vaginal misoprostol in pregnancies uncomplicated with risk factors such as premature rupture of membranes and non-cephalic presentation. While this study included several outcomes, the lack of incorporation of intrapartum and maternal outcomes was a notable limitation. As there are limited studies on higher doses of misoprostol, future studies should compare 50 mcg vaginal misoprostol with other cervical ripening methods and labor induction. Further investigation is also needed to delineate and characterize the efficacy and safety of this dose in shorter intervals.