1. Background
Infertility is defined as not being able to conceive after 12 months of regular and unprotected intercourse. It affects approximately 10 - 15% of women of reproductive age (1) and is considered a major healthcare problem worldwide. Infertility can affect one or both partners. The main causes are anatomical, physiological, and genetic factors, although the etiology is unknown in close to 30% of the cases (2). Many environmental contaminants, such as chemical agents in water, food, and health-and-beauty aids, may affect reproductive function (3). This damage not only decreases normal fertility but also makes in vitro fertilization (IVF) much less likely to succeed (4).
Parabens (PBs) are para-hydroxybenzoic acid esters that have been used as preservatives for over 70 years, mainly in personal care products, medicines, and food. They are mainly used for their antibacterial and antifungal properties. The most active metabolites are methylparaben (MP) (5), ethylparaben (EP), propylparaben (PP), butylparaben (BP), and heptylparaben (2, 6). Parabens are easily absorbed through the skin (7) and are excreted in the urine shortly after entering the body (8).
Parabens are allowed to be added as food preservatives due to the Food and Drug Administration’s (FDA) permission. According to the agreement of the experts of the Joint Committee on Food Additives of the Food and Agriculture Organization and the World Health Organization (JECFA/WHO) in 1974, the acceptable total absorption of methyl, ethyl, and PP is less than 10 mg/kg per day (9).
Recent studies have debated the safety of PBs (10, 11) due to a significant relationship between urinary PB concentration and oxidative stress biomarkers in pregnant women (12) or sperm DNA damage (13). Therefore, an accurate evaluation of the PB effect on the human endocrine system is of particular importance (14). Parabens can mimic the human hormone estrogen regardless of gender. Low micromolar concentrations of hexyl- and heptyl PB decreased 17 beta-hydroxysteroid dehydrogenase 1 activity, while EP and ethyl vanillate decreased 17 beta-hydroxysteroid dehydrogenases 2 activity which is important in estradiol synthesis (15). Indeed, recent evidence provides an association between infertility and high levels of PBs in women (16).
2. Objectives
Due to the increasing rate of using cosmetics and processed foods and the fact that there is currently no tight control over the amount of PB in these products, this study investigated the relationship between the urinary concentration of PB derivatives, including MP (5), EP, PP, and BP, with sex hormones and lifestyle components in infertile women and compared them with controls. We also evaluated the relationship between PB derivatives, hormonal profile, and IVF outcomes in the infertile group.
3. Methods
3.1. Setting
This case-control study was conducted in the Vali-e-Asr Hospital affiliated with the Tehran University of Medical Sciences between March and December 2021. All women of reproductive age who attended the infertility clinic and have lived in Tehran for more than 1 year were asked to participate in this study. For the infertile group the criteria were: (1) women between 18 and 48 years old; (2) women who hadn’t gotten pregnant after at least a year of unprotected, regular intercourse; and (3) women who are candidates for IVF for reasons other than tubal or uterine problems. The research excluded couples with male factor infertility or female factor infertility owing to tubal or uterine causes. In addition, this study excluded women having a history of chronic conditions or long-term drug usage. The normal group was randomly selected among the women who were referred to the gynecology clinic for any conditions other than infertility. Informed consent was obtained from all participants.
3.2. Data Gathering Form
Participants were asked to fill out a questionnaire about themselves, their history of infertility, whether or not they smoked, and how often they used processed foods, personal care products (PCPs), cosmetics, detergents, vitamins and supplements. After two weeks, the above goods were put into three groups based on how often they were used: Low (0 - 3 products), medium (4 - 7 products), and high (8 - 19 products). Also, in the group of infertile patients, the correlation between the hormonal profile, including follicle stimulating hormone (FSH), luteinizing hormone (LH), anti-mullerian hormone (AMH), thyroid stimulating hormone (TSH), anti-thyroid peroxidase antibodies (anti-TPO), and estradiol (E2), and IVF outcomes (the number and quality of oocytes and embryos) with urinary PBs was investigated and reported.
3.3. Ethical Considerations
This study was approved by the review boards of the Tehran University of Medical Sciences under reference number IR.TUMS.IKHC.REC.1400.033. The protocol of the study was designed according to the ethical principles of the Declaration of Helsinki. All participants agreed to participate in the study, and written informed consent was obtained from all cases.
3.4. Urine Analysis
All cases were referred to the central lab of the Vali-e-Asr Hospital for a urine test. They collected 50 cc of urine samples in a sterile polypropylene container. After measuring the specific gravity (SG) using a hand refractometer, urine samples were frozen at -20° Celsius (C) in a dark place and sent to the referral laboratory. Parabens were analyzed by gas chromatography-mass spectrometry (GC-MS/MS). Standard stock solutions (1 mg/ml) were prepared from each PB metabolite in acetonitrile, one for amplifying quality control urine samples and the other for calibration.
The analysis was performed by gas chromatography (Varian GC-450) equipped with a VF5-ms capillary column (30 m × 0.25 mm × 0.25 μm + 10 m EZ protector, Varian) and split/split injector 1177 (isothermal conditions: 280°C). Stove column program: 60°C (3 minutes), 60°C - 140°C (120°C per minute), 140°C - 290°C (17°C per minute), 280°C (13 minutes). The detection limit of MP, EP, and PP was 0.5 ng/mL and for BP was 1.0 ng/mL. The values for PBs were adjusted for urinary creatinine concentration.
3.5. Statistical Analysis
Statistical analysis was conducted using SPSS (version 27.0, SPSS Inc., Chicago, IL, USA). Descriptive statistics were presented as mean ± standard deviation (SD) for the normal distribution of numeric variables and frequency (percentage) for categorical variables. The chi-square test or Fisher’s exact test was utilized for the comparison of the qualitative variables. The geometric mean, arithmetic mean, SD, 10, 25, 50, 75, and 90 percentiles, as well as the minimum and maximum values of PBs, were summarized. In univariate analysis, the exact Mann-Whitney U test was used to compare the median of PBs between the infertile and control groups. Simple linear regression and an analysis of variance were used to evaluate the impact of PBs in numeric and quartile forms on parameters. P values less than 0.05 were considered statistically significant.
4. Results
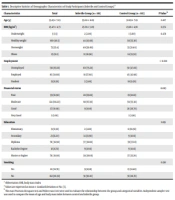
A total of 206 women were included in the study, 101 of whom were categorized as infertile and 105 as control subjects. The demographic and lifestyle characteristics are listed in Table 1. Accordingly, the relation between the two groups (infertile and control) and variables including employment and financial status were significant. The distribution of urinary PB concentrations was shown in Table 2. The geometric mean and minimum of all variables were zero. The means of MP, EP, PP, and BP and the comparison of the urine PB derivatives between groups are summarized in Table 3, and the differences were not significant.
| Characteristics | Total | Infertile Group (n = 101) | Control Group (n = 105) | P-Value b |
|---|---|---|---|---|
| Age (y) | 33.83 ± 7.03 | 35.02 ± 6.69 | 32.69 ± 7.19 | 0.017 |
| BMI (kg/m2) | 25.47 ± 4.73 | 25.78 ± 5.09 | 25.18 ± 4.36 | 0.372 |
| Underweight | 3 (1.5) | 2 (2.00) | 1 (1.00) | 0.478 |
| Healthy weight | 100 (48.5) | 44 (43.60) | 56 (53.30) | |
| Overweight | 73 (35.4) | 40 (39.60) | 33 (31.40) | |
| Obese | 25 (12.1) | 11 (10.90) | 14 (13.30) | |
| Employment | < 0.001 | |||
| Unemployed | 130 (63.10) | 80 (79.20) | 50 (47.60) | |
| Employed | 63 (30.60) | 18 (17.80) | 45 (42.90) | |
| Student | 13 (6.30) | 3 (3.00) | 10 (9.50) | |
| Financial status | 0.003 | |||
| Poor | 39 (18.90) | 20 (19.80) | 19 (18.10) | |
| Moderate | 124 (60.20) | 68 (67.30) | 56 (53.30) | |
| Good | 37 (18.00) | 9 (8.90) | 28 (26.70) | |
| Very good | 2 (1.00) | 2 (1.90) | ||
| Education | 0.119 | |||
| Elementary | 13 (6.30) | 2 (2.00) | 11 (10.50) | |
| Secondary | 23 (11.20) | 14 (13.90) | 9 (8.60) | |
| Diploma | 76 (36.90) | 37 (36.60) | 39 (37.10) | |
| Bachelor degree | 18 (8.70) | 9 (8.90) | 9 (8.60) | |
| Master or higher | 76 (36.90) | 39 (38.60) | 37 (35.20) | |
| Smoking | 0.501 | |||
| Yes | 22 (10.70) | 9 (8.90) | 13 (12.40) | |
| No | 182 (88.30) | 91 (90.10) | 91 (86.70) |
Abbreviation: BMI, body mass index.
a Values are expressed as mean ± standard deviation or No. (%).
b The exact Pearson chi-square test and Fisher exact test were used to evaluate the relationship between the group and categorical variables. Independent sample t-test was used to compare the mean of age and body mass index between control and infertile group.
| Parameter | Geometric Mean | Mean ± SD | Minimum | Percentiles | Maximum | ||||
|---|---|---|---|---|---|---|---|---|---|
| 10 | 25 | 50 | 75 | 95 | |||||
| Methylparaben | 0 | 14.75 ± 22.20 | 0 | 0 | 0.29 | 7.05 | 14.33 | 74.12 | 96.62 |
| Ethylparaben | 0 | 17.67 ± 25.60 | 0 | 0 | 0.00 | 9.60 | 21.45 | 78.84 | 118.27 |
| Propylparaben | 0 | 7.29 ± 14.48 | 0 | 0 | 0.00 | 0.00 | 6.70 | 35.95 | 73.62 |
| Butylparaben | 0 | 57.13 ± 42.12 | 0 | 0 | 29.82 | 51.62 | 87.35 | 128.00 | 180.97 |
a Data are presented as mean ± standard deviation, range.
| Paraben Derivatives a | Univariate b | Paraben Derivatives | Multivariate c | ||||
|---|---|---|---|---|---|---|---|
| Infertile Group (n = 101) | Control Group (n = 105) | P-Value | Infertile Group (N = 101) | Control Group (n = 105) | P-Value | ||
| Methylparaben | 6.34 (0.70, 15.13) | 7.33 (0.00, 13.49) | 0.772 | Log Methylparaben | 2.07 ± 1.45 | 2.37 ± 1.31 | 0.212 |
| Ethylparaben | 9.90 (0.00, 23.60) | 8.83 (0.00, 20.83) | 0.708 | Log Ethylparaben | 2.55 ± 1.29 | 2.62 ± 1.13 | 0.840 |
| Propylparaben | 0.00 (0.00, 10.27) | 0.00 (0.00, 6.15) | 0.318 | Log Propylparaben | 2.32 ± 1.25 | 2.22 ± 0.86 | 0.863 |
| Butylparaben | 51.05 (27.94, 87.35) | 52.92 (31.92, 86.36) | 0.840 | Log Butylparaben | 4.03 ± 0.81 | 4.02 ± 0.84 | 0.819 |
a The provided values are adjusted for urinary creatinine and are expressed as μg/g.
b The exact Mann-Whitney U test was used to compare the median of parabens between infertile and control groups.
c The ANOVA was used to compare the mean of the log transformation of each paraben between the infertile and control groups adjusting for age.
We inquired about the 23 PCPs and cosmetics that the participants had most used in the last two weeks. The frequency and comparison of used personal care products, cosmetics, detergents, and foodstuffs in infertile and control groups within a 2-week period are listed in Appendices 1, 2, and 3, respectively. The general use of personal care products and cosmetics was found to be higher in the control group, and although most differences were insignificant, certain product usages, including hair conditioner, hair care lotion, makeup remover, and cosmetic foundation, were significantly higher in the control group. Similar to PCPs and cosmetic products, foods containing preservatives also had a higher level of consumption among the control group, while, acid folic was more frequently used in the cases.
Table 4 summarizes the effects of PBs on various parameters, including FSH, LH, AMH, TSH, anti-TPO, and E2. As compared to the first quartile, a significant effect of MP on E2 was observed for the second, third, and fourth quartiles. Follicle stimulating hormone was significantly affected by the fourth quartile of EP. Additionally, the effect of EP on AMH was negative and significant for the fourth quartile compared to the first quartile. There was a significant impact of PP on FSH, LH, AMH, and E2.
| Type of Paraben a | FSH | LH | AMH | TSH | AntiTPO | E2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef | 95%CI | P | Coef | 95%CI | P | Coef | 95%CI | P | Coef | 95%CI | P | Coef | 95%CI | P | Coef | 95%CI | P | |
| MP | ||||||||||||||||||
| Cont. | -0.04 | -0.11, 0.03 | 0.302 | -0.02 | -0.09, 0.06 | 0.599 | -0.02 | -0.06, 0.01 | 0.215 | 0.01 | -0.01, 0.02 | 0.263 | 1.19 | -0.01, 2.38 | 0.051 | 0.29 | -3.88, 4.46 | 0.877 |
| Q2 | -2.74 | -8.32, 2.85 | 0.325 | -0.34 | -6.24, 5.55 | 0.906 | 2.55 | -0.41, 5.50 | 0.089 | 0.01 | -1.13, 1.15 | 0.988 | -89.00 | -238.85, 60.85 | 0.232 | -607.17 | -693.53, -520.80 | < 0.001 |
| Q3 | -2.94 | -8.09, 2.21 | 0.253 | 0.05 | -5.38, 5.49 | 0.984 | 1.58 | -1.13, 4.29 | 0.243 | 0.33 | -0.75, 1.41 | 0.541 | -38.61 | -169.03, 91.81 | 0.547 | -598.00 | -689.60, -506.40 | < 0.001 |
| Q4 | -3.63 | -8.78, 1.52 | 0.160 | 1.99 | -3.45, 7.42 | 0.461 | 1.71 | -1.00, 4.42 | 0.209 | -0.26 | -1.34, 0.81 | 0.621 | -59.04 | -192.68, 74.61 | 0.371 | -574.14 | -656.07, -492.21 | < 0.001 |
| EP | ||||||||||||||||||
| Cont. | 0.07 | 0.00, 0.13 | 0.038 | -0.03 | -0.10, 0.05 | 0.478 | -0.02 | -0.05, 0.02 | 0.313 | 0.00 | -0.01, 0.02 | 0.787 | 0.04 | -1.19, 1.27 | 0.944 | -0.93 | -12.04, 10.17 | 0.854 |
| Q2 | -0.81 | -5.63, 4.00 | 0.732 | -2.29 | -7.71, 3.14 | 0.393 | -0.89 | -3.05, 1.27 | 0.404 | 0.08 | -0.96, 1.12 | 0.875 | 64.48 | -52.97, 181.94 | 0.266 | -303.77 | -826.22, 218.69 | 0.182 |
| Q3 | -2.24 | -7.37, 2.89 | 0.378 | -3.36 | -9.14, 2.41 | 0.242 | -0.35 | -2.65, 1.95 | 0.757 | -0.12 | -1.23, 0.99 | 0.825 | 5.01 | -112.44, 122.46 | 0.930 | -301.75 | -874.07, 270.57 | 0.217 |
| Q4 | 5.66 | 0.01, 11.31 | 0.050 | -2.08 | -8.44, 4.29 | 0.508 | -2.81 | -5.21, -0.41 | 0.023 | 0.34 | -0.77, 1.45 | 0.537 | 0.47 | -127.37, 128.31 | 0.994 | -324.70 | -1025.65, 376.25 | 0.268 |
| PP | ||||||||||||||||||
| Cont. | -0.06 | -0.17, 0.06 | 0.298 | -0.04 | -0.16, 0.07 | 0.451 | 0.02 | -0.04, 0.08 | 0.493 | 0.01 | -0.02, 0.03 | 0.491 | -0.01 | -1.81, 1.80 | 0.994 | 0.72 | -7.88, 9.31 | 0.854 |
| Q2 | -2.49 | -7.12, 2.13 | 0.278 | -1.67 | -6.93, 3.60 | 0.521 | 2.32 | 1.77, 2.87 | < 0.001 | -45.19 | -139.52, 49.15 | 0.331 | 165.33 | -282.18, 612.84 | 0.386 | 9.48 | 5.66, 13.29 | < 0.001 |
| Q3 | -3.20 | -8.49, 2.08 | 0.223 | 1.80 | 0.60, 3.00 | 0.005 | -0.04 | -0.94, 0.85 | 0.920 | -33.65 | -146.85, 79.56 | 0.544 | 0.00 | 0.00, 0.00 | < 0.001 | -3.39 | -9.22, 2.44 | 0.242 |
| Q4 | 6.20 | 3.32, 9.08 | < 0.001 | 2.00 | 0.08, 3.92 | 0.042 | -0.26 | -1.23, 0.71 | 0.584 | 51.27 | -287.02, 389.55 | 0.713 | 0.00 | 0.00, 0.00 | < 0.001 | -3.59 | -8.99, 1.81 | 0.183 |
| BP | ||||||||||||||||||
| Cont. | 0.00 | -0.04, 0.04 | 0.977 | 0.00 | -0.05, 0.04 | 0.937 | -0.01 | -0.03, 0.01 | 0.392 | 0.00 | -0.01, 0.01 | 0.650 | 0.32 | -0.40, 1.04 | 0.368 | 1.51 | -1.19, 4.22 | 0.238 |
| Q2 | -3.39 | -9.22, 2.44 | 0.242 | 2.05 | -3.82, 7.92 | 0.479 | 0.73 | -1.75, 3.20 | 0.553 | 0.59 | -0.46, 1.64 | 0.262 | -3.33 | -124.64, 117.98 | 0.955 | -1.70 | -838.30, 834.90 | 0.996 |
| Q3 | -3.59 | -8.99, 1.81 | 0.183 | 4.18 | -1.26, 9.61 | 0.126 | 1.56 | -0.83, 3.96 | 0.191 | 0.06 | -0.96, 1.07 | 0.910 | 5.91 | -115.40, 127.22 | 0.920 | 15.25 | -667.83, 698.33 | 0.954 |
| Q4 | -4.56 | -10.14, 1.03 | 0.106 | 0.50 | -5.12, 6.12 | 0.856 | 0.30 | -2.17, 2.78 | 0.803 | -0.43 | -1.45, 0.58 | 0.389 | 78.49 | -46.96, 203.94 | 0.207 | 197.50 | -426.07, 821.07 | 0.429 |
Abbreviations: MP, methylparaben; EP, ethylparaben; PP, propylparaben; BP, butylparaben; Cont., continuous variable.
a Reference groups 1. In case Q2, Q3, Q4 reference is Q1; Q1 ≤ 25 percentile; Q2 (25–50] percentile; Q3 (50–75] percentile; Q4 > 75 percent
Also, the correlation of IVF outcome with urinary paraben levels is listed in Table 5, and no significant correlation was found.
| Paraben Derivatives | AFC | M2 | M1 | GV | Embryo Grade A | Embryo Grade B | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | β | P | β | P | |
| Methylparaben | 0.434 | 0.210 | 0.446 | 0.120 | 0.324 | 0.099 | -0.101 | 0.479 | 0.504 | 0.109 | 0.276 | 0.172 |
| Ethylparaben | 0.037 | 0.851 | -0.105 | 0.603 | 0.041 | 0.840 | 0.411 | 0.133 | -0.104 | 0.613 | -0.094 | 0.648 |
| Propylparaben | 0.037 | 0.851 | -0.080 | 0.692 | -0.340 | 0.083 | -0.114 | 0.572 | 0.035 | 0.864 | -0.043 | 0.834 |
| Butylparaben | 0.40 | 0.841 | -0.040 | 0.834 | 0.173 | 0.389 | 0.173 | 0.388 | -0.079 | 0.700 | 0.040 | 0.847 |
Abbreviations: M1, metaphase I; M2, metaphase II; GV, germinal vesicle.
5. Discussion
In this study, we found that urinary PB levels, including MP, EP, PP, and BP, were not significantly different among fertile and infertile women. Also, it was revealed that the PB concentration had no positive correlation with hormonal distribution and IVF outcomes.
The daily exposure to PBs among the rodents resulted in disturbances in ovarian folliculogenesis and decreased early primary follicles (17). This was proved in another study on humans that assessed couples’ urinary concentrations of PB in the context of fecundity and concluded that the women’s preconception urinary concentrations of MP and EP were associated with a reduction in the couple’s pregnancy rate (18).
Hajizadeh et al. measured urinary PB concentrations in 95 Iranian pregnant women to assess the amount of PB consumption and determine the factors that affected the rate of exposure to this chemical compound (5). They showed that among pregnant women in Iran, urine concentrations of MP, EP, and PP, with the exception of BP, are similar to those of other countries. Also, the highest daily intake was related to MP (5). Contrariwise, in this study, BP had the highest amount of consumption; however, BP concentration wasn’t significantly higher in infertile women than in the control group.
In a study by Smith et al. the effect of urinary PB concentrations on ovarian aging was evaluated. It was indicated that there wasn’t a significant correlation between urinary MP or BP and day-3 FSH or AFC. Also, the ovarian volume wasn’t associated with urinary MP, PP, or BP, but it was demonstrated that PP may be related to decreased ovarian reserve (10). The results are mostly similar to ours. We did not find a significant relationship between AMH, FSH, LH, e2 and PB concentrations in a short duration; we may need longer follow-up to reach a more accurate conclusion.
Inconsistent with our findings, Minguez-Alarcon et al. proved that the urinary PB concentrations were not related to oocyte counts, good-quality embryos, and fertilization rates in infertile women (19). In addition, they showed that the outcomes of infertility treatments and the rate of live birth are not affected by urinary PB levels (19).
In contrast with our findings, Jurewicz et al. found that chronic exposure to PP (no other PB compounds) may impair fertility by reducing antral follicle count E2 and increasing FSH concentration (20). This result may show that the PB compounds, in the long term, may have destructive effects on ovarian reserves and fertility, which requires more detailed long-term studies.
The study’s strength is in its follow-up and evaluation of the results for infertile couples using assisted reproductive technologies (ART). The status of PB pollution may be different in every country and city, which depends on lifestyle and different environmental and consumption variables. This is the first study that evaluated many factors of the lifestyle of fertile and infertile women. Our clinical goal is to detect the main sources of PB and help women live a healthier lifestyle. Due to the low resource allocation for this study, we could not repeat measuring urine, and this result awaits corroboration by further cohort studies. However, longer follow-up and evaluating the effect of PBs on male fertility are needed to be investigated as well.
5.1. Conclusions
Urinary PB levels may not be an indicator for infertility and hormonal distribution, at least in the short term, and also did not affect the IVF results, which require further investigation.