1. Background
Premature preterm membrane rupture (PPROM) with a 3% prevalence refers to fetal membrane rupture before the 37th week and the onset of uterine contractions (1). A history of PPROM, reproductive system infection, antepartum bleeding, and smoking are important risk factors for this phenomenon (2). About one-third of PPROM cases are associated with the risk of maternal infection, including chorioamnionitis, endometritis, and septicemia (3, 4). Inflammation in the amniotic fluid and placenta, known as chorioamnionitis, is responsible for neonatal complications related to PPROM (5). Placental abruption occurs in PPROM with a 2 - 5% prevalence, which is 7 - 9 times higher in the cases of chorioamnionitis and oligohydramnios.
The non-cephalic presentation prevalent in PPROM is associated with a higher risk of umbilical cord prolapse, infection, and fetal mortality (6). Fetal and neonatal complications include mortality, asphyxia, sepsis, premature birth, septic shock, pneumonia, meningitis, pulmonary dysplasia, intraventricular hemorrhage, damage to the brain white matter, and cerebral palsy. These are the complications of preterm birth as a result of PPROM and have a higher occurrence in the cases of chorioamnionitis (7-12).
Neonatal sepsis is a clinical syndrome occurring in infants younger than 28 days, specified with a range of systemic symptoms of infection and positive blood culture (13). Early-onset neonatal sepsis refers to the onset of symptoms before 72 hours, often due to the transmission of infection from the birth canal or infected amniotic fluid (14). Maternal chorioamnionitis is the most well-known risk factor for early-onset neonatal sepsis. Other risk factors are fever equal to or higher than 38°C in the mother, gestational age of < 37 weeks, breakage of the amniotic sac for more than 18 hours, and group B strep infection (15-17). Numerous studies have been conducted in recent years to identify markers predicting chorioamnionitis infection and neonatal sepsis, with different or sometimes contradictory results. There are numerous reports on the importance of C-reactive protein (CRP) levels in chorioamnionitis diagnosis (5).
2. Objectives
The platelet count (PLT), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) have not been used to diagnose chorioamnionitis, and it is used as a non-invasive and inexpensive marker of sub-clinical chorioamnionitis. We aimed to examine the relationship between maternal NLR, PLR, and PLT inflammatory factors to diagnose chorioamnionitis.
3. Methods
This cohort study was conducted at Shahid Akbar-Abadi Training Hospital, Tehran, Iran. Patients visiting this hospital diagnosed with leakage before < 34 weeks were hospitalized and included in this study. The exclusion criteria entailed multiple pregnancies, uterine malformation, polyhydramnios, diabetes, preeclampsia, immunologic disorders, antepartum bleeding, maternal systemic infections (e.g., urinary tract infection and pneumonia), meconium in the amniotic fluid, and clinical chorioamnionitis at the time of admission. Gestational age was < 34 weeks based on the last day of menstruation or the first-trimester ultrasound results. Leakage was confirmed by examination using a sterile speculum or AmniSure test. Before starting antibiotics and corticosteroids, a maternal blood sample was sent for complete blood count (CBC) and differential count (Diff). Antibiotics included IV ampicillin for two days, oral amoxicillin for five days, and dexamethasone injection (12 mg) once daily for two days. Another blood sample was sent for CBC-Diff at the termination of pregnancy. During hospitalization, the status of the mother and fetus was closely evaluated. The evaluation included a chart of vital signs (especially the mother’s body temperature and heart rate), fetus’s heart rate (every 4 hours), daily abdominal examination in terms of tenderness and uterine contractions, examination of uterine malodorous and purulent discharge twice a week, daily non-stress test (NST), and weekly ultrasound. Based on a 1-year census, the files belonging to all patients were examined. The data recorded in these files were studied using a questionnaire, including a data collection sheet. The data were collected individually for each participant. The study started after receiving official approval from the Iran University of Medical Sciences and submitting it to the Dean of Shahid Akbar Abadi Training Hospital. The present study was conducted according to the Declaration of Helsinki, and all patients were informed of the research process and received all the necessary information at every step. Moreover, this study was discussed and approved by the Department of Gynecology. The data were collected using a researcher-made questionnaire and analyzed using SPSS version 16 (SPSS Inc., Chicago, Ill, USA). The frequency was calculated for qualitative variables. In addition, mean, range and standard deviation (SD) were calculated for quantitative variables, and P-values less than 0.05 were considered statistically significant. To examine NLR, PLR, and PLT, a t-test or its non-parametric equivalent was used. Moreover, the chi-square test was used to examine quantitative variables, and the receiver operating characteristic (ROC) curve analysis was performed to find the cut-off point for the emergence of different causes of termination.
4. Results
In this study, 110 patients were examined, of whom 30 had given birth before the 34th week (27.3%), 19 had chorioamnionitis (17.3%; mean age of 27.7 years), 32 had pain and dilations (29.1%; mean age of 29.2 years), and 29 were hospitalized for other reasons (26.4%; mean age of 32.9 years). The mean age did not significantly differ regarding causes (P = 0.06). The mean parameters in CBC per cause are presented in Table 1. In CBC, only NLR significantly differed between the four groups (Table 1).
| Groups | PLT | NLR | PLR | PLT | NLR | PLR |
|---|---|---|---|---|---|---|
| P-value | 0.348 | 0.251 | 0.768 | 0.546 | 0.007 | 0.942 |
| Week 34 | 230.9 ± 60.2 | 3.768 ± 1.37 | 101.12 ± 55.8 | 226.63 ± 53.2 | 3.78 ± 2.044 | 81.83 ± 27.4 |
| Chorioamnionitis | 216.1 ± 85.7 | 4.378 ± 2.37 | 99.17 ± 31.69 | 212.53 ± 45.3 | 5.48 ± 2.145 | 81.15 ± 31.9 |
| Pain and dilation | 240.6 ± 58.9 | 3.434 ± 1.39 | 92.12 ± 32.93 | 233.69 ± 57.3 | 3.78 ± 1.733 | 80.64 ± 30.3 |
| Other | 248.7 ± 56.6 | 3.537 ± 1.75 | 91.97 ± 36.39 | 234.14 ± 63.2 | 3.64 ± 1.896 | 77.16 ± 33.7 |
Abbreviations: PLT, platelets; NLR, neutrophil-to-lymphocyte; PLR, platelet-to-lymphocyte.
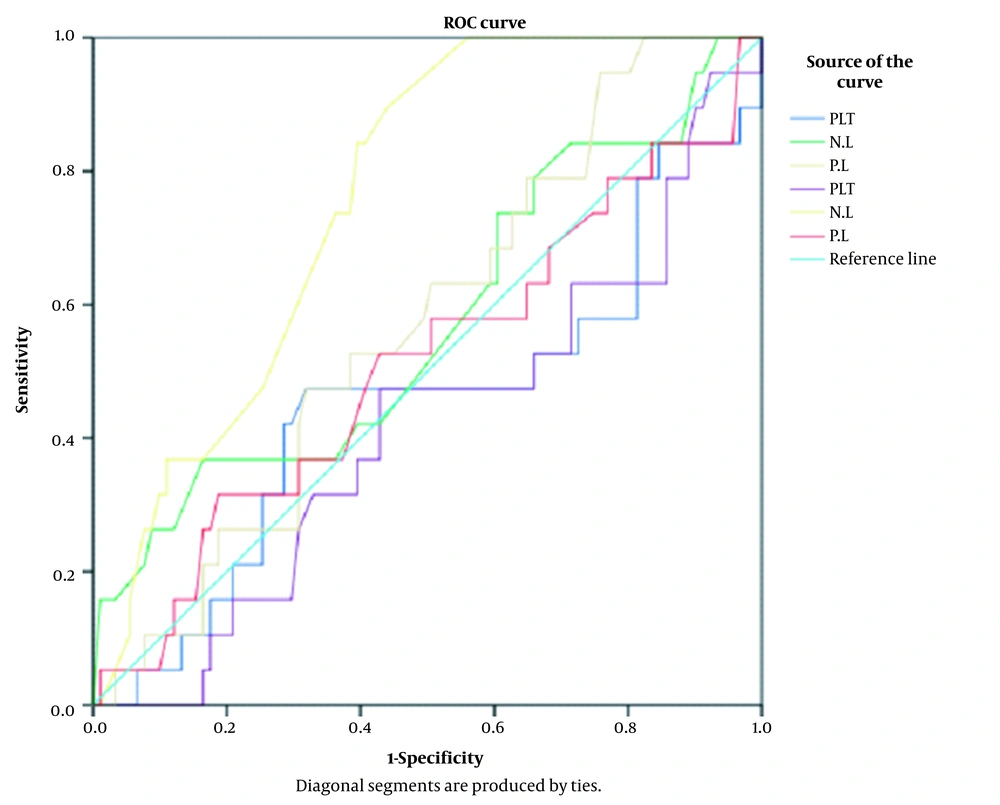
In this study, the NLR value at the cut-off points of 2.3-5.3 had an acceptable sensitivity with a low specificity for identifying cases with chorioamnionitis (Figure 1, Table 2).
| Test Result Variables | Area | Std. Error | Cut of Point | Sensitivity | 1 - Specificity | Asymptotic P-Value | Asymptotic 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| NLR | 0.751 | 0.055 | 3.23 | 0.867 | 0.430 | 0.002 | 0.643 | 0.858 |
| 3.33 | 0.800 | 0.405 | ||||||
| 3.55 | 0.800 | 0.392 | ||||||
Abbreviation: NLR, neutrophil-to-lymphocyte.
Differences between the first and second CBC parameters in patients with chorioamnionitis showed that the level of neutrophils could indicate inflammation and infection (Table 3).
| Cause of Termination | WBC 1 | NUT 1 | WBC 2 | NUT 2 |
|---|---|---|---|---|
| Chorioamnionitis | 18.98 ± 3.78 | 72.25 ± 10.9 | 20.55 ± 4.9 | 81.2 ± 2.5 |
Abbreviations: WBC, white blood cell; NUT, neutrophil.
5. Discussion
The main finding of our study was that NLR was significantly higher in the PPROM with chorioamnionitis group compared to the cases without chorioamnionitis. Chorioamnionitis is the bacterial intra-amniotic infection of the fetal membrane (amnion and chorion). It is usually due to the bacteria from the vagina to the uterus and is related to long work hours. Chorioamnionitis occurs in 2% of births in the USA and is one of the causes of preterm birth. In general, 1 - 4% of all births in the USA are coupled with chorioamnionitis. The prevalence of chorioamnionitis is related to diagnostic criteria, known risk factors, and gestational age (3-8). Chorioamnionitis is associated with 40% of neonatal sepsis cases. It is also a risk factor for long-term neurodevelopmental disorders. Ten percent of women with chorioamnionitis have a positive bacterial blood test, including Group B Streptococcus (GBS) and Escherichia coli (1-5).
Subclinical chorioamnionitis is an important clinical problem in obstetrics (18). Placental histology is the gold standard for diagnosing chorioamnionitis and has a role in guiding the postnatal care of the newborn. It is used and available after delivery. Maternal serological markers are inexpensive and easily accessible (19). In general, an intra-amniotic inflammatory response is shown by different markers, such as white blood cell count, IL-6 in amniotic fluid, obtained, and matrix metalloproteinase-8 (MMP-8) by amniocentesis (20).
PLR is an important marker in predicting thrombotic events, inflammatory diseases, and malignancies (21). However, reducing the time between diagnosis and cesarean delivery in adjusting broad-spectrum antibiotics does not improve the final results (8-14). Recent studies have discussed the faster diagnosis of this condition. Numerous studies in recent years have been conducted to identify markers predicting chorioamnionitis infection and neonatal sepsis, showing different or sometimes contradictory results. There are several reports on the importance of CRP levels in diagnosing chorioamnionitis (5).
A retrospective study by Yoon et al. found that CRP and WBC did not have a high predictive power for the diagnosis of chorioamnionitis (22). Nevertheless, according to Popowski et al., CRP equal to or above 5 was related to chorioamnionitis and neonatal sepsis (23). In addition, Buhimschi et al. predicted the amniotic liquid, umbilical cord inflammation, and early-onset neonatal sepsis by amniocentesis and evaluated human neutrophil defensin and calgranulin A and C biomarkers (24). Moreover, Yücel and Ustun used NLR, PLR, and PLT as inflammatory factors to measure the severity of preeclampsia (25).
Soykan Sert and Bülbül showed that PLR and NLR values were higher in women with preterm than those with term labor (26). In another study, some clinicians showed that PLR increased in patients with premature rupture of membranes, and they recommended this parameter as a new inflammation marker (21). Ekin et al. found a relationship between PLR and PPROM regarding the latency period. They did not observe a significant difference between PRL and latency periods < 72 hours and > 72 hours (27). Mahmoud showed that PLR and NLR are important predictive markers for early-onset neonatal sepsis (28).
The PLT, NLR, and PLR have not been used in diagnosing chorioamnionitis and its neonatal complications. In this study, NLR at the cut-off points of 2.3 - 5.3 had an acceptable sensitivity and a low specificity for identifying cases with chorioamnionitis. The NLR is a simple ratio obtained from CBC routinely performed during pregnancy. A high NLR can indicate the risk of placental inflammation, possibly a simple diagnostic warning for possible infection in women without signs or symptoms. Future trials can be conducted to evaluate and investigate its ability to predict desired pregnancy outcomes. This study showed that inflammatory markers in maternal blood have diagnostic value and are important for pregnant women at risk of premature birth and infection of mother and baby.
5.1. Study Limitations
There were some limitations to the present study. First, this study had a small sample size. Second, we did not have NLR in the first trimester of pregnancy.