1. Background
The annual incidence rate of the ovarian dermoid cyst or mature cyst teratoma is 10 in 100,000 women (1). Ovarian cancer is the fifth leading cause of cancer-related deaths in women due to a lack of diagnostic tools resulting in late diagnosis (2, 3). Adnexal masses have different etiologies. They may present with various symptoms, such as abdominal and pelvic pain, bloating, or increasing abdominal size (4). Most ovarian cysts are benign (5), and the most common one is the dermoid cyst or mature cystic teratoma (MCT) (6).
Mature cystic teratomas are more common in reproductive age (7). They may present with different findings in imaging, including Rokitansky nodules, calcifications, palm tree-like protrusion, diffuse or regional high amplitude echoes called intratumoral fat, and dot-dash sign (8). The modality choice of diagnosis is transvaginal ultrasonography (9-11). Surgical resection of the cyst is often the treatment of choice, especially in uncomplicated cases. The incidence of malignant transformation in mature cystic teratoma has been reported to be 1 - 3%, mostly occurring in postmenopausal women (12, 13).
Tumor markers such as CA19-9 and CA125 may be used to manage ovarian cancer (14). CA125 is the best and one of the most common (15) tumor markers for ovarian cancer. However, diagnosing ovarian cancer is a combination of physical examination, ultrasonography, and CA125 measurement (16, 17). CA125 and CA19-9 can be helpful in diagnosing mature cystic teratoma (18). These markers may also be useful for malignancy prediction in some borderline tumors alongside other tumor markers like HE4, NLR (19, 20), and CEA (21).
2. Objectives
The aim of this study was to assess the role of tumor markers CA19-9 and CA125 in diagnosing the dermoid cyst before surgery and distinguishing it from the malignant type in Yas Hospital from 2019 to 2021.
3. Methods
We conducted a case-control study in patients with adnexal mass in Yas Hospital, Tehran, Iran, from 2019 to 2021. The study was approved by the Ethics Committee of Tehran University of Medical Sciences. The sample size for each group was calculated to be 80, using the reported values in previous studies10, the study power of 80%, and the two-tailed significance level of 5%. Patients’ information was collected considering ethical aspects using patient file numbers without mentioning names. The cyst size and being unilateral or bilateral, was determined with ultrasound imaging. Transabdominal (3.5 to 5 MHz) and, if needed, transvaginal ultrasonography (5 to 7 MHz transducer) were performed in one center by an attending radiologist. The level of tumor markers was also measured in the laboratory of Yas Hospital using blood serum or plasma. Tumor markers were determined by radioimmunoassay. The cut-off values for CA19-9 and CA125 were 37 and 35 U/mL, respectively. Patients’ symptoms were collected based on what they expressed in their history. Statistical analyses were done with SPSS-25 (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp; 2017). The results were stated as frequency (%) or mean ± standard deviation (SD) for categorical and numerical variables. We used median and range for data that were not normally distributed. Chi-square or Fisher’s exact tests were used for comparisons. The mean differences in numerical variables between the two groups were compared by independent t-test. For numerical variables, Pearson’s correlation and linear regression were used. A P-value of less than 0.05 was considered statically significant.
4. Results
After pathology confirmation postoperatively, 95 cases with dermoid cyst and 85 cases with ovarian cancer were included. The average age of patients with the dermoid cyst was 33.23 ± 5.23 years, and the average age of ovarian cancer was 36.24 ± 4.53 years. Patients with dermoid cyst were significantly younger than malignant cases (P-value < 0.001). Our investigation of the symptoms at the first visit showed that abdominal pain was the most common symptom in the dermoid cyst group; however, the most frequent symptom was pelvic pain in the malignancy group (Table 1). The mean cyst diameter was 4.25 ± 1.31 cm in dermoid cysts and 8.56 ± 1.82 cm in malignant mass. Cyst diameter was significantly larger in malignant cases (P-value = 0.023).
| Symptoms | Dermoid Cyst | Ovarian Cancer | P-Value |
|---|---|---|---|
| Abdominal pain | 57 (59.6) | 77 (90) | < 0.001 |
| Menstrual disorders | 21 (21.7) | 7 (9) | < 0.001 |
| Incidental | 17 (18.7) | 1(1) | < 0.001 |
a Values are expressed as No. (%).
The mean levels of CA19-9 and CA125 in the patients with dermoid cysts were 32.26 ± 5.45 units/mL and 31.37 ± 4.77 units/mL, respectively. More than 51% of them had a high level of CA19-9. In the malignant ovarian tumor group, the mean level of CA19-9 and CA125 was 9.56 ± 3.54 units/mL and 233.79 ± 54.57 units/mL, respectively. Interestingly, more than 77% of these patients had a high level of CA125. CA19-9 level was significantly higher in the dermoid cyst group (P-value < 0.001). CA125 level was significantly elevated in the malignant group (P-value < 0.001) (Table 2).
| Ovarian Dermoid Cyst (units/mL) (N = 95) | Ovarian Cancer (units/mL) (N = 85) | P-Value | |
|---|---|---|---|
| CA19-9 | 32.26 ± 5.45 | 9.56 ± 3.54 | < 0.001 |
| CA125 | 31.37 ± 4.77 | 233.79 ± 54.57 | < 0.001 |
a Values are expressed as mean ± SD.
One of the factors expected to affect the tumor marker level is the presence of a mass in both ovaries simultaneously. For this reason, we separated the unilateral and bilateral cases. In the dermoid cyst group, 38 cases (35.2%) were right-sided, 49 cases (45.4%) were left-sided, and eight patients (19.4%) were bilateral. In the ovarian cancer group, 70 cases (82.4%) were one-sided, and the remaining cases were bilateral. Cyst location was not significantly different between both groups (P-value = 0.477).
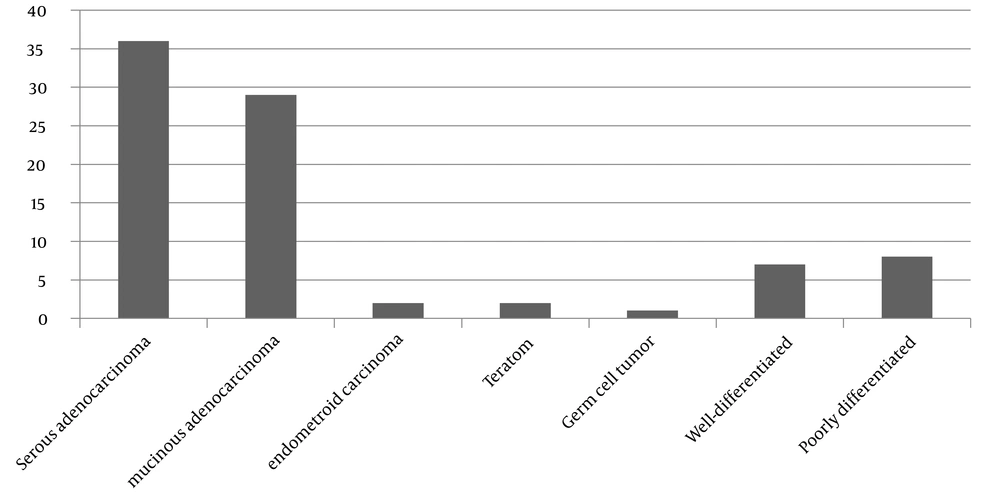
Among the cancerous patients, 36 people have serous adenocarcinoma, 29 people have mucinous adenocarcinoma, two people have endometroid carcinoma, two people have teratoma, one person has germ cell tumor, seven people have well-differentiated adenocarcinoma, and eight people have poorly differentiated carcinoma (most of the patients in this group are epithelial type) (Figure 1).
In the correlation coefficient analysis, we measured the age and the level of tumor markers based on the symptoms, then whether the cyst was unilateral or bilateral. It was observed that there was a significant relationship between the level of CA19-9 with the size of the cyst (r = 0.192, P = 0.007) and the level of CA125 with the age of the patients (r = -0.237, P = 0.005). In the ANOVA analysis, no significant relationship was found between the level of tumor markers and whether they were unilateral or bilateral.
5. Discussion
Mature teratoma cyst or dermoid cyst is the most common teratoma, and ovarian neoplasm (10 - 25%) constitute 60% of all benign ovarian neoplasms (22). They are mostly unilateral (23). In our study, 80.6% of dermoid cysts were unilateral. This cyst grows slowly and is bilateral in 10% of cases (24). Malignant changes in this type of ovarian cyst are in about 1 - 2% of patients, and torsion is their most complication (25). Some radiologic features, like ultrasonography, can suggest MCT, but tumor markers alongside the imaging accommodate the diagnosis (8). The majority of signs and symptoms of ovarian cancer are nonspecific. Abdominal pain was the most frequent symptom (59.6%), followed by menstrual disorders in dermoid cyst cases in our study.
Lack of specific and sensitive biomarkers can lead to late diagnosis and even patient death (26). It showed that the mean level of CA19-9 was higher in the dermoid group than in the cancer group, and the mean level of CA125 was much lower in the dermoid group than in the cancer group. It is similar to Wang et al.’s study at Wenzhou Medical University. They found that CA19-9 was likely the most reliable tumor marker for diagnosing MCT (27). It also resembles Mughir and Al-Hilli’s study in 2019 in Iraq on 50 cases of ovarian dermoid cysts and 50 control patients. Their results show that the CA19-9 level in the case group was about four times higher than in the control group. In our study, there was a direct relationship between the size of the cyst and the level of CA19-9; likewise, in their study, there was a direct relationship between the size of the tumor and the level of CA19-9. In their study, most dermoid cysts are unilateral, and about 10 - 15% of them were bilateral, as well as our research (28).
In Prodromidou et al.’s study and colleagues, the meta-analysis showed that in patients with elevated CA19-9, there was an increased size in mature cystic teratoma. At the same time, there was no relationship between the patient’s age, bilaterality, site of the lesion, and the simultaneous elevation of CA-125 in patients with elevated CA19-9 (29). It is consistent with our findings that there was no correlation between CA19-9 and age or being unilateral/bilateral, but there is a significant correlation with size. In Lertkhachonsuk et al.’s study, CA125 was the best diagnostic tumor marker for borderline and malignant mucinous ovarian tumors, followed by CA19-9 and CEA (30). This is similar to our study that in the cancer group, 88% of patients have elevated CA125. Ali et al.’s study demonstrated that the combination of microRNA-204, CA125, and CA19.9 was the best test for the early detection of ovarian tumors and cancers, but in our study, elevated CA19-9 was more related to the dermoid cyst group and elevated CA125 more related to cancer group (26). Also, in Bagde et al.’s study LDH, BHCG, and CA19-9 did not significantly differ in malignant and benign cases (31).
In Sagi-Dain et al.’s study, they demonstrated that combining CA19-9 with CA125 compared with CA125 alone did not significantly affect sensitivity and specificity in differentiating malignant from benign adnexal masses. The mean CA19-9 levels were higher in metastatic carcinoma and could help differentiate metastatic tumors from primary ovarian malignancy (32). The strength point of this study is that all imaging assessments and tumor marker measurements are done in an academic referral center. Increased sample size and assessment of other tumor markers can be suggested for future studies to cover the weak points of our study. Most of the dermoid cysts in the present study were unilateral. According to the relationship of CA19-9 in the dermoid cyst group with cyst size, it can be said that the larger the cyst size, the higher the tumor marker level will be. The increase in the level of CA125 suggests the possibility of malignancy in the ovarian cyst alongside an increase in age and cyst size. Finally, CA19-9 can be used in diagnosis along with other diagnostic tools, especially ultrasound. This tumor marker has no diagnostic value in small cysts, but if the cyst size increases, it will help in a definitive diagnosis.
5.1. Conclusions
Regarding the correlation between the cyst size and CA19-9 by comparison of these two groups, this tumor marker may be a suitable diagnostic marker in benign ovarian cysts along with ultrasound. High levels of CA125 will be in favor of the malignant type.