1. Background
Nuchal translucency (NT) refers to the subcutaneous space in the fetal neck and can be measured by ultrasound imaging in the first trimester (1-3). As an important marker for prenatal screening, NT thickness increases with gestational age and crown-rump length (2, 4-6). Increased NT thickness in the first trimester was associated with a higher risk of chromosomal abnormalities, genetic syndromes, congenital heart defects, structural abnormalities, intrauterine infection, delayed nerve growth, and fetal mortality (7).
Although the pathophysiology of the increase in NT thickness is still unclear, the possible explanations for fluid accumulation, including heart failure, venous congestion in the head and neck, altered extracellular matrix composition, abnormal growth or growth retardation of the lymphatic system, and lymphatic drainage failure (8-11).
Otherwise, it has not yet been clear whether the serum biochemical, drug intake or assisted reproductive techniques (ART) potentially affect NT thickness or possibly lead to an increase in false-negative or false-positive rates in Down syndrome screening. For example, first-trimester screening in ART should be based on maternal age and NT, as bio-markers are significantly altered in these women (12), or anemia may be associated with increased NT (13). Based on these data, we hypothesize that maternal markers may be associated with changes in NT or CRL.
2. Objectives
The purpose of this study was to determine the serum levels of ferritin, hemoglobin, hematocrit, fasting blood sugar, vitamin D, and thyroid-stimulating hormone during the first trimester, as well as investigate the relationship between these levels and fetal NT thickness and crown-rump length (CRL).
3. Methods
3.1. Ethical Consideration
This study was approved by the Tehran University of Medical Sciences ethics committee, and patient records were reviewed. All participants read and signed the informed consent. This study was conducted according to the Helsinki declarations.
3.2. Study Setting
This cross-sectional study was performed in an academic hospital from March 2020 to March 2022. Patients screened for Down syndrome at 11 - 13 weeks of gestation were selected to participate in the study. Initial screening was done by measuring NT. Inclusion criteria were women screened for Down syndrome in 11 - 13 weeks of pregnancy. The exclusion criteria were incomplete patient records.
Serum levels of ferritin, hemoglobin, hematocrit, fasting blood sugar, vitamin D, and TSH were measured at 11 to 13 weeks of gestation. Demographic information was also recorded, including age, weight, height, maternal drug history, and pregnancy with In vitro fertilization (IVF). The patient's family and obstetrics history were also recorded. Fetal NT and CRL were also recorded at 11 to 13 weeks of gestation.
The thickness of fetal NT at 11 - 13 weeks of gestation and CRL were measured according to Fetal Medicine Foundation recommendations, using the trans-abdominal method at the sagittal level where the fetus fills three-quarters of the image. All obstetricians used the Philips Affinity 70 system with a 3 - 5 MHz transducer. During the NT measurement, a complete fetal scan was performed to determine if there were any structural abnormalities in the fetus.
3.3. Statistical Analysis
Descriptive statistics were presented using mean±standard deviation (SD) for symmetric numerical variables. Asymmetric data were summarized by the median (interquartile range [IQR]). Frequency (percentage) was used to represent categorical variables. A correlogram was used to show the relationship between numerical variables and NT. Independent t-test and analysis of variance (ANOVA) were used to compare the mean of NT between levels of variables. Simple linear regression was used to evaluate the impact of variables on the NT measurements. All analyses were performed using SPSS (version 16). P-values less than 0.05 were considered statistically significant.
4. Results
The 258 cases participated in the study with a mean ± SD age of 32.61 ± 5.2 years. The average values of BMI (26.19), FBS (87.54), vitamin D (29.74), and Hgb (13.04) are reported in Table 1. The median values for TSH, FR, HCT, and CRL were 2.1, 29.8, 38.9, and 60.0, respectively.
| Variables | Total Cases (n = 258) |
|---|---|
| BMI, kg/m2 | 26.19 ± 4.29 |
| Age, y | 32.61 ± 5.20 |
| FBS, mg/dL | 87.54 ± 9.79 |
| VitD, ng/mL | 29.74 ± 13.51 |
| TSH, mU/L | 2.10 (1.29, 3.13) |
| FR, ng/mL | 29.85 (18.00, 47.15) |
| HCT, % | 38.90 (37.20, 40.50) |
| Hgb, g/dL | 13.04 ± 1.09 |
| CRL, mm | 60.00 (55.15, 64.00) |
| IVF | |
| Negative | ---- |
| Positive | 4 (1.60) |
| PG | |
| Negative | ---- |
| Positive | 47 (18.20) |
| Age of NT (mm), y | |
| 11 | 30 (11.60) |
| 12 | 185 (71.70) |
| 13 | 43 (16.70) |
Abbreviations: BMI, body mass index; FBS, fasting blood sugar; VitD, serum vitamin D; TSH, thyroid-stimulating hormone; FR, serum ferritin; HCT, hematocrit; Hgb, hemoglobin; CRL, crown-rump length; IVF, in vitro fertilization; PG, pregnancy.
a The mean ± standard deviation (SD) was used for symmetric numerical data. Asymmetric data were summarized using the median (interquartile range [IQR]). Nominal data were represented using the frequency (percentage).
According to the correlogram, the correlation coefficient between NT and CRL was 0.28 (P < 0.05). In addition, a significant correlation was observed between FBS and variables, including BMI (r = 0.13) and TSH (r = 0.20). Other variables were not correlated and were shown by cross lines. (Figure 1)
In the next step, the distribution of NT was represented in different levels of variables. Results showed that the mean NT thickness increased significantly from 1.53 mm when CRL measurement was < 49 mm to 1.83mm with ≥ 70 mm-sized CRL (P-value < 0.001). The greatest thickness of NT was detected at 13 weeks of gestation, significantly different from that at 11 weeks (P-value = 0.028). No significant relationship was observed between the distribution of NT and VitD (P-value = 0.212), FR (P-value = 0.790), and Hgb (P-value = 0.469) (Table 2).
| Variables | No. (%) | NT, mm | P-Value | |
|---|---|---|---|---|
| Mean ± SD | Median (IQR) | |||
| Serum vitamin D, ng/mL | 0.212 | |||
| Deficient (< 20) | 61 (32.62) | 1.71 ± 1.91 | 1.46 (0.37, 1.70) | |
| Insufficient (20 – 29.9) | 8 (4.28) | 1.44 ± 1.60 | 1.23 (0.24, 1.47) | |
| Normal (≥ 30) | 118 (63.10) | 1.71 ± 1.96 | 1.40 (0.45, 1.70) | |
| Serum ferritin, ng/mL | 0.790 | |||
| Hypoferritinemia (< 30) | 110 (49.55) | 1.71 ± 1.91 | 1.47 (0.40, 1.70) | |
| Normal ferritin (≥ 30) | 112 (50.45) | 1.72 ± 1.95 | 1.40 (0.43, 1.70) | |
| Hemoglobin, g/dL | 0.469 | |||
| Anemia (< 11) | 9 (3.54) | 1.61 ± 0.37 | 1.70 (1.30, 1.90) | |
| Normal (≥ 11) | 245 (96.46) | 1.71 ± 0.43 | 1.70 (1.41, 1.95) | |
| Crown-rump length, mm | < 0.001 | |||
| < 49 | 20 (7.87) | 1.53 ± 0.40 | 1.47 (1.17, 1.76) | |
| 50 - 59 | 107 (42.13) | 1.61 ± 0.34 | 1.60 (1.40, 1.81) | |
| 60 - 69 | 113 (44.49) | 1.83 ± 0.46 | 1.80 (1.60, 2.10) | |
| ≥ 70 | 14 (5.51) | 1.83 ± 0.49 | 1.78 (1.50, 2.27) | |
| Age of nuchal translucency, w | 0.028 | |||
| 11 | 29 (11.37) | 1.61 ± 1.83 | 1.35 (0.44, 1.58) | |
| 12 | 183 (71.76) | 1.69 ± 1.93 | 1.41 (0.39, 1.70) | |
| 13 | 43 (16.86) | 1.86 ± 2.23 | 1.60 (0.53, 1.79) | |
As shown in Table 3, the impact of the numerical type of CRL on the NT was significant (β = 0.01, 95% CI: 0.00, 0.02, P-value = 0.002). Furthermore, categorical CRL significantly impacted the NT at levels 60-69 vs. < 49 (β = 0.30, 95% CI: 0.10, 0.49, P-value = 0.003) and ≥ 70 vs. < 49 (β = 0.30, 95% CI: 0.02, 0.58, P-value = 0.037). Gestational age significantly affected NT in weeks 13 vs. 11 (β = 0.25, 95% CI: 0.05, 0.45, P-value = 0.015).
| Variables | Β (95% CI) | P-Value |
|---|---|---|
| Body mass index, kg/cm2 | -0.00 (-0.01, 0.01) | 0.891 |
| Age, y | 0.005 (-0.01, 0.02) | 0.346 |
| Fast blood sugar | 0.00 (-0.00, 0.01) | 0.595 |
| Serum vitamin D | 0.00 (-0.00, 0.01) | 0.280 |
| Insufficient vs. deficient | -0.27 (-0.57, 0.04) | 0.090 |
| Normal vs. deficient | -0.00 (-0.13, 0.13) | 0.987 |
| Thyroid-stimulating hormone | -0.02 (-0.05, 0.01) | 0.118 |
| Serum ferritin | 0.00 (0.00, 0.00) | 0.159 |
| Normal ferritin vs. hypoferritinemia | 0.02 (-0.10, 0.13) | 0.790 |
| Hematocrit | 0.00 (-0.01, 0.02) | 0.591 |
| Hemoglobin | 0.03 (-0.02, 0.08) | 0.213 |
| Anemia vs. normal | -0.11 (-0.39, 0.18) | 0.469 |
| Crown-rump length | 0.01 (0.00, 0.02) | 0.002 |
| 50 - 59 vs. < 49 | 0.08 (-0.12, 0.28) | 0.426 |
| 60 - 69 vs. < 49 | 0.30 (0.10, 0.49) | 0.003 |
| ≥ 70 vs. < 49 | 0.30 (0.02, 0.58) | 0.037 |
| Gestational age of nuchal translucency, w | ||
| 12 vs. 11 | 0.08 (-0.08, 0.249) | 0.319 |
| 13 vs. 11 | 0.25 (0.05, 0.45) | 0.015 |
5. Discussion
In this study, NT and CRL values at 11 - 13 weeks of gestation were independent of maternal hemoglobin, FBS, vitamin D3, and ferritin status. These values have been demonstrated to screen for fetal abnormalities, aneuploidy, and perinatal outcomes. Although, spurious association and confounding effects of other laboratory parameters during pregnancy may skew these parameters and lead to false screening results.
Additionally, several markers, including vitamin D and maternal iron levels, have been demonstrated to be associated with fetal development in preclinical studies (14, 15). It was indicated that Vitamin D and classical functions have an immunological role that affects placental growth (16, 17). Also, iron has an important role as a cofactor in enzymatic reactions and affects fetal brain development (18). However, the impact of these factors has not been evaluated to a satisfactory extent in observational studies on humans. This study investigated the association of Vitamin D, FBS, TSH, ferritin status, and maternal hematocrit with NT thickness and CRL. The results of this study did not demonstrate any significant correlations between the laboratory and ultrasound markers.
Vitamin D insufficiency is frequent among pregnant women. Also, low maternal vitamin D level is associated with poor pregnancy outcomes, including preeclampsia, fetal growth restriction, and preterm birth, but the underlying mechanism for these correlations is still unclear (19, 20). As per our findings, Fernandez-Alonso et al. conducted a cross-sectional study on 498 pregnant women and found no correlation between first-trimester NT and CRL measurements and maternal serum 25-hydroxyvitamin D levels (21). Many maternal factors may affect the total l 25(OH)D, such as smoking, maternal age, BMI, and ethnicity. So, insufficient corrections for these factors could influence our findings (22).
Previous studies revealed that o maternal thyroid function is key in fetal brain development (23, 24). The lack of association between TSH and ultrasound parameters in this study could be attributed to the insignificant role of TSH in regulating thyroid function compared to the more prominent and overlapping role of beta-hCG (25, 26). Hantoushzadeh et al. evaluated 643 pregnant women to determine the correlation between maternal thyroid hormones and NT thickness. They did not report any association between TSH and NT, despite significant correlations between maternal thyroxine and NT thickness. These results were demonstrated to be independent of CRL (27).
Poor iron status is related to adverse pregnancy outcomes such as low birthweight, preterm birth, and intrauterine growth restriction. Although we have not found any significant relationship between ferritin status and NT thickness, Kosus et al., by comparing screening markers between pregnant women with ferritin levels < 15 and > 15 µg/L, demonstrated a significant difference in pregnancy-associated plasma protein-A (PAPP-A) and free β-human chorionic gonadotropin (FB-hCG) between the two groups (28). In this era, choosing different cut-off values for ferritin may be responsible for these conflicting results.
A retrospective investigation by Savvidou et al. has studied the correlation between the first-trimester screening parameters for chromosomal abnormalities and the different types of diabetes during pregnancy. Consistent with our findings, they showed that the NT thickness and maternal beta-hCG level were not changed in pregnant women with pre-existing diabetes or who subsequently developed GDM (29). In addition, Leipold et al. revealed that NT thickness was not altered in pregnant women with glucose disorders since using NT measurement for the risk assessment of aneuploidy does not require to be adjusted for glucose level (30).
The major limitation of this study was the limited sample size to rule out potential small effect sizes and correlations with the evaluated markers. Nevertheless, our results are supported by several previous studies evaluating the association of biochemical factors with NT thickness at distinct gestational stages of pregnancy (27, 28). Also, we suggest that further studies with large sample sizes are required to evaluate NT thicknesses in diabetic mothers and hypothyroid pregnant women and compare the measurements with normal pregnant women.
Most cases with NT thickness between 2.5 - 3.5 mm and normal karyotype are also born as neonates without adverse perinatal outcomes (21). Moreover, normal NT measurements are observed in a significant portion of Down syndrome (31), which altogether, the association of these markers with fetal abnormalities. It is worth noting that while the current study did not reveal an association between study variables and ultrasound markers, the importance of operator-dependent accuracy of NT measurement should not be neglected to achieve the highest detection rate for fetal abnormalities (32).
5.1. Conclusions
In 11 - 13 weeks of gestation, NT and CRL values are independent of maternal hemoglobin, FBS, vitamin D3, and ferritin status. However, future large-scale studies should incorporate the number of abnormalities evaluated postnatally.
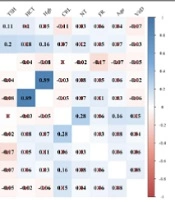
![The correlogram of NT with other variables [crosslines determined non-significant correlations]. BMI, body mass index; FBS, fasting blood sugar; VitD, serum vitamin D; TSH, thyroid-stimulating hormone; FR, serum ferritin; HCT, hematocrit; Hgb, hemoglobin; CRL, crown-rump length. The correlogram of NT with other variables [crosslines determined non-significant correlations]. BMI, body mass index; FBS, fasting blood sugar; VitD, serum vitamin D; TSH, thyroid-stimulating hormone; FR, serum ferritin; HCT, hematocrit; Hgb, hemoglobin; CRL, crown-rump length.](https://services.brieflands.com/cdn/serve/3170b/5e0399f23267f0d43d19e2ebec6ead57ac1b9d97/fga-138371-i001-F1-preview.webp)