1. Background
The coronavirus disease 2019 (COVID-19) pandemic has had a significant impact on maternal health, leading to clinical and psychological complications and changes in healthcare provision and accessibility to healthcare services (1). Pregnant women with COVID-19 infection experience higher rates of hospitalization and admission to the intensive care unit (ICU) (2). This could be attributed to pregnancy-induced alterations in the immune system and the body's response to viral infections during fetal development (3).
In non-pregnant patients with COVID-19 infection, a massive release of pro-inflammatory cytokines and chemokines can lead to a cytokine storm, triggering severe inflammatory responses and causing tissue/organ damage or even death (4). Consequently, a short course of glucocorticoids has been shown to improve clinical outcomes in patients with severe COVID-19 (5). Additionally, there is evidence that COVID-19 infection significantly affects the cytokine profile in pregnant women, with cytokine levels correlating with disease severity and varying across trimesters (6).
Although COVID-19 primarily impacts the respiratory system, there have been reports of metabolic disturbances, hyperglycemia, and new-onset diabetes shortly after the pandemic's outbreak (7, 8). One possible explanation is that the SARS-CoV-2 virus may affect pancreatic β-cells, reducing insulin secretion. Furthermore, the significant production of cytokines can induce insulin resistance. Both reduced insulin secretion and increased insulin resistance can contribute to hyperglycemia, regardless of previous diabetes status (9, 10).
Pregnancy is associated with relative glucose intolerance and insulin resistance. Therefore, the combination of pregnancy and corticosteroids may lead to impaired glycemic control in both diabetics and non-diabetics. The duration and severity of hyperglycemia were significantly associated with the initial glucose tolerance test before corticosteroid therapy and other maternal risk factors for gestational diabetes mellitus (GDM) (11).
Long-term use of corticosteroids during pregnancy is linked to adverse effects for both the fetus and the mother, including hyperglycemia (12), preeclampsia, preterm premature rupture of membranes, pyelonephritis, and thromboembolisms. Neonates born to mothers who have received corticosteroid therapy during pregnancy are more susceptible to hypoglycemia (13). Additionally, short-term antenatal corticosteroid therapy is commonly administered to women at risk of preterm delivery to promote fetal pulmonary maturation. Previous studies have recommended monitoring glycemic levels for at least 5 days after initiating corticosteroid therapy to detect any potential hyperglycemia (14, 15).
As a result, corticosteroids may lead to impairments in blood sugar control and other adverse effects in pregnant women with moderate to severe COVID-19. Several studies have investigated corticosteroid therapy in pregnant women with COVID-19 disease (16, 17). However, we did not find any studies specifically addressing the impact of corticosteroid therapy on the incidence of hyperglycemia and insulin requirements in pregnant women with COVID-19 infection.
2. Objectives
The objective of this study was to compare 2 different corticosteroid therapies, namely systemic dexamethasone, and methylprednisolone, in terms of their effects on glycemic status during pregnancy and the duration of hyperglycemia until the postpartum period.
3. Methods
3.1. Setting
This cohort study was conducted from August 2021 to November 2021 at an academic center. The inclusion criteria comprised pregnant women with COVID-19 (singleton pregnancies) confirmed by real-time polymerase chain reaction (PCR) who required hospitalization. Admission criteria were defined as moderate to severe illness based on the National Institutes of Health (NIH) criteria, including SpO2 < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mm Hg, a respiratory rate > 30 breaths/min, or lung infiltrates > 50% (18).
Exclusion criteria encompassed gestational or overt diabetes mellitus, hypertension, prior corticosteroid use for any reason, contraindications to steroid therapy, immunodeficiency disorders, and unwillingness to participate in the study. Additionally, the diagnostic criteria for GDM included at least 1 abnormal value (≥ 92, 180, and 153 mg/dL for fasting, 1-hour, and 2-hour plasma glucose concentration, respectively) following a 75 g oral glucose tolerance test (OGTT) (19), which was conducted for all participants.
3.2. Study Design
The admitted patients received treatment and monitoring from a team of healthcare providers, including infectious disease specialists and obstetricians. Each participant was treated according to 1 of the 2 hospital protocols. Protocol 1 involved administering 2 mg/kg of methylprednisolone intravenously (IV) daily (infused over 60 minutes), which was tapered to half the dosage every 5 days for a total of 10 days. Protocol 2 consisted of 6 mg of dexamethasone intravenously daily for 10 days. Both protocols included the administration of remdesivir, with 200 mg IV on the first day followed by 100 mg IV daily for 5 days, and anticoagulation therapy with enoxaparin or heparin, which was common to both protocols.
Fasting and postprandial plasma glucose levels at 2 hours after each meal were evaluated 24 hours after corticosteroid therapy. Fasting sugar levels above 92 and postprandial levels above 120 were considered indicative of hyperglycemia. If 30% of the glucose levels were above normal within 48 hours, insulin treatment and a diabetic diet were initiated for the patients. Blood glucose levels were monitored until hyperglycemia was under control. Fetal monitoring was conducted using the fetal biophysical profile (BPP) twice weekly, and fetal growth was assessed by ultrasound every 3 weeks as long as hyperglycemia persisted. After discharge, glucose control was continued, and insulin administration was adjusted if necessary until normal glucose levels were achieved.
Demographic features, as well as baseline clinical and laboratory characteristics, oxygen saturation, type of oxygen supplementation, and respiratory rate, were evaluated in all patients. All participants were followed up until 6 weeks after delivery, and their glycemic status, gestational age at delivery, mode of delivery, and duration of insulin requirement were assessed and compared between the 2 groups.
3.3. Statistical Analysis
All analyses were performed using SPSS v. 26.0 (IBM Corp., Armonk, NY, USA). Numerical data were described as mean ± standard deviation (SD), and categorical data were presented as numbers (%). Statistical differences were assessed using an independent t-test or Mann-Whitney U test as appropriate. The chi-square test or Fisher's exact test was used for comparing categorical variables. The paired-sample t-test was employed to evaluate changes in clinical indices before and after the intervention, with a P-value < 0.05 considered statistically significant.
3.4. Ethical Considerations
The study received approval from the ethics committee of Tehran University of Medical Sciences with an institutional review board (IRB) number IR.TUMS.IKHC.REC.1400.351. Written informed consent was obtained from all patients or their legally authorized representatives after providing an explanation of the study's objectives and methods to the best of their understanding.
4. Results
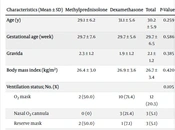
A total of 75 pregnant women were assessed, of which 59 met the inclusion criteria. Among these, 26 (44.1%) patients received methylprednisolone, while 33 (55.9%) patients received dexamethasone. As shown in Table 1, there were no significant differences (P > 0.05) in demographic and clinical characteristics between the 2 groups at the time of admission.
| Characteristics (Mean ± SD) | Methylprednisolone | Dexamethasone | Total | P-Value c |
|---|---|---|---|---|
| Age (y) | 29.1 ± 6.2 | 31.1 ± 5.6 | 30.2 ± 5.9 | 0.259 |
| Gestational age (week) | 29.7 ± 7.6 | 29.7 ± 5.6 | 29.7 ± 6.5 | 0.586 |
| Gravida | 2.3 ± 1.2 | 1.9 ± 1.2 | 2.1 ± 1.2 | 0.385 |
| Body mass index (kg/m2) | 26.4 ± 3.0 | 26.9 ± 3.6 | 26.7 ± 3.4 | 0.420 |
| Ventilation status; No. (%) | 0.105 | |||
| O2 mask | 2 (50.0) | 10 (71.4) | 12 (20.3) | |
| Nasal O2 cannula | 0 (0) | 3 (21.4) | 3 (5.1) | |
| Reserve mask | 2 (50.0) | 1 (7.1) | 3 (5.1) | |
| Pulmonary involvement in CT scan (%) | 29.8 ± 13.1 | 31.7 ± 15.8 | 30.9 ± 14.6 | 0.672 |
a Data are presented as mean ± SD unless otherwise indicated.
b Mean ± SD compared by Mann-Whitney U test. No. (%) compared by the chi-square test.
c Significant level was considered P < 0.05.
The laboratory indices at admission for each group are displayed in Table 2. There were no statistically significant differences in laboratory indices between the groups (P-value > 0.05), except for D-dimer, which was significantly higher in the methylprednisolone group (P-value = 0.033). However, it is important to note that there was no evidence of thromboembolism reported in the patients.
| Characteristics | Methylprednisolone | Dexamethasone | Total | P-Value b |
|---|---|---|---|---|
| FBS | 104.2 ± 11.6 | 102.6 ± 15.4 | 103.3 ± 13.8 | 0.340 |
| WBC | 8.4 ± 2.8 | 7.3 ± 2.5 | 7.7 ± 2.7 | 0.240 |
| Neutrophil | 6.6 ± 2.1 | 5.7 ± 2 | 6.1 ± 2.1 | 0.151 |
| Lymphocyte | 1.8 ± 0.9 | 1.6 ± 0.7 | 1.7 ± 0.8 | 0.783 |
| Lymphocyte/Neutrophil ratio | 0.27 ± 0.1 | 0.29 ± 0.1 | 0.28 ± 0.1 | 0.436 |
| Hemoglobin | 11.6 ± 1.2 | 12 ± 2 | 11.9 ± 1.7 | 0.448 |
| Platelet | 198.1 ± 63.7 | 188.4 ± 56.5 | 192.7 ± 59.5 | 0.531 |
| ESR | 85.9 ± 40.9 | 86.2 ± 31.1 | 86.1 ± 35.4 | 0.691 |
| CRP | 73.7 ± 36.3 | 75.5 ± 23.2 | 74.7 ± 29.5 | 0.502 |
| Creatinine | 0.7 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.220 |
| AST | 33.8 ± 14.4 | 32 ± 9.8 | 32.8 ± 12 | 0.915 |
| ALT | 34 ± 12.9 | 32.2 ± 9.8 | 33 ± 11.2 | 0.903 |
| LDH | 38 ± 12.5 | 36.7 ± 10.3 | 37.3 ± 11.3 | 0.888 |
| Ferritin | 153.2 ± 181.1 | 109.6 ± 76.4 | 128.3 ± 131.7 | 0.492 |
| D-dimer | 1352 ± 2512.3 | 75 ± 362.5 | 591.9 ± 1715.3 | 0.033 |
Abbreviations: FBS, fasting blood sugar (glucose); WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine transaminase; LDH, lactate dehydrogenase.
a Data are presented as mean ± SD. Mean ± SD compared by the Mann-Whitney U test.
b Significant level was considered P < 0.05.
In total, 24 (40.7%) cases developed hyperglycemia during hospitalization, requiring insulin control. The incidence of increased glucose levels in the methylprednisolone and dexamethasone groups was 8 (30.8%) and 16 (48.5%), respectively, showing no significant difference (P-value = 0.169). A comparison of patient characteristics between the hyperglycemic and normal groups before starting treatment is presented in Table 3. Additionally, a comparison of the patients' laboratory indices between the hyperglycemic and normal groups is shown in Table 4. As observed, there were no differences in patient characteristics and laboratory indices, except for higher fasting blood glucose (P-value < 0.000) in the hyperglycemic group.
| Characteristics | Glucose Intolerance | Normal | P-Value b |
|---|---|---|---|
| Age (y) | 30.5 ± 6.5 | 30.0 ± 5.5 | 0.610 |
| Gestational age (week) | 30.4 ± 3.9 | 29.2 ± 7.8 | 0.770 |
| Gravida | 2.1 ± 1.4 | 2.1 ± 1.1 | 0.790 |
| Body mass index (kg/m2) | 26.5 ± 2.9 | 26.8 ± 3.6 | 0.798 |
| O2 saturation | 94.4 ± 2.1 | 94.7 ± 2.2 | 0.651 |
| Ventilation status; No. (%) | 0.504 | ||
| O2 mask | 3 (50.0) | 9 (75.0) | |
| Nasal O2 cannula | 1 (16.7) | 2 (16.7) | |
| Reserve mask | 2 (33.3) | 1 (8.3) | |
| Pulmonary involvement in CT scan | 34.0 ± 16.7 | 28.7 ± 12.8 | 0.296 |
Abbreviation: CT, computed tomography.
a Data are presented as mean ± SD. Mean ± SD compared by the Mann-Whitney U test.
b Significant level was considered P < 0.05.
| Characteristics | Glucose Intolerance | Normal | P-Value b |
|---|---|---|---|
| FBS | 111.6 ± 13.5 | 97.3 ± 10.7 | < 0.000 |
| 2-hour postprandial glucose (morning) | 110.4 ± 7.6 | 110.3 ± 8.3 | 0.614 |
| 2-hour postprandial glucose (midday) | 120.0 ± 10.3 | 109.5 ± 7.6 | 0.705 |
| 2-hour postprandial glucose (afternoon) | 124.5 ± 27.2 | 116.0 ± 9.1 | 0.614 |
| WBC | 7.1 ± 1.9 | 8.2 ± 3 | 0.151 |
| Neutrophil | 5.6 ± 1.6 | 6.4 ± 2.3 | 0.123 |
| Lymphocyte | 1.5 ± 0.6 | 1.7 ± 0.9 | 0.758 |
| Hemoglobin | 11.5 ± 1.3 | 12.1 ± 1.9 | 0.215 |
| Platelet | 207.6 ± 72.8 | 182.5 ± 46.7 | 0.547 |
| ESR | 90.2 ± 24.8 | 83.2 ± 41.3 | 0.090 |
| CRP | 77.6 ± 24.6 | 72.7 ± 32.6 | 0.294 |
| Creatinine | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.212 |
| AST | 33 ± 8.7 | 32.7 ± 13.9 | 0.371 |
| ALT | 33.7 ± 9.7 | 32.5 ± 12.2 | 0.371 |
| LDH | 38.3 ± 11.7 | 36.6 ± 11.1 | 0.613 |
| Ferritin | 176.3 ± 188.7 | 95.7 ± 56.4 | 0.124 |
| D-dimer | 159 ± 476.5 | 916.6 ± 2195.6 | 0.247 |
Abbreviations: FBS, fasting blood glucose; WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine transaminase; LDH, lactate dehydrogenase.
a Data are presented as mean ± SD. Mean ± SD compared by the Mann-Whitney U test.
b Significant level was considered P < 0.05.
During follow-up telephone calls, 47 out of 59 (79.6%) of the patients responded. Preterm delivery (P-value = 1.00) and Cesarean section (P-value = 0.617) in patients with and without hyperglycemia did not show significant differences. This result was consistent across both treatment groups, including methylprednisolone (P-value = 0.638) and dexamethasone (P-value = 0.404). In the hyperglycemia group, the duration of insulin requirement lasted up to 4 weeks and did not differ significantly between the 2 treatment groups (Table 5).
| Insulin Requirement | Methylprednisolone | Dexamethasone | P-Value |
|---|---|---|---|
| During hospitalization | 1 (16.6) | 4 (44.4) | 0.611 |
| 1 week after discharge | 2 (33.3) | 2 (22.2) | 0.847 |
| 10 days after discharge | 0 | 1 (11.1) | 0.409 |
| 2 weeks after discharge | 0 | 1 (11.1) | 0.409 |
| 4 weeks after discharge | 3 (50.0) | 1 (11.1) | 0.633 |
a Data are presented as No. (%). No. (%) compared by the chi-square test or Fisher's exact test.
5. Discussion
This study revealed that the incidence of increased blood glucose after treatment with corticosteroids in pregnant women with moderate to severe COVID-19 infection was 40.7%, requiring insulin control. Although serum glucose levels returned to normal within 4 weeks, no significant difference was observed between the 2 corticosteroid therapy regimens.
COVID-19 has been shown to potentially disrupt metabolism in the long term, leading to increased blood sugar levels and the onset of diabetes. This effect may be attributed to the virus's binding to angiotensin-converting enzyme 2 (ACE2), which is present in pancreatic beta cells and adipose tissue. It is likely that SARS-CoV-2 can directly impair glucose metabolism (20). Additionally, the inflammatory response triggered by the infection can contribute to insulin resistance and dysfunction of beta cells (21).
In non-pregnant cases with severe COVID-19 infection, the administration of systemic corticosteroids has been associated with lower mortality (4). This is achieved by ameliorating lung inflammation and preventing acute respiratory distress syndrome (22), as well as reducing the duration of mechanical ventilation (23). However, the use of corticosteroids in treating acute COVID-19 can exacerbate insulin resistance. Additionally, SARS-CoV-2 may worsen the existing pro-inflammatory state seen in type 2 diabetes. Glycemic abnormalities have been observed in hospitalized COVID-19 patients for up to 2 months after their initial diagnosis (24). These findings emphasize the importance of continuous monitoring and management of blood sugar levels in individuals who have recovered from COVID-19.
While corticosteroids are not entirely safe during pregnancy, they are recommended for pregnant women with moderate-to-severe COVID-19. In cases where oral prednisolone or intravenous hydrocortisone is not an option, methylprednisolone can be considered (25). The World Health Organization (WHO) makes a similar recommendation, though it does not explicitly specify which corticosteroid should be administered to women with moderate-to-severe COVID-19 (26).
Pregnancy is known to induce significant acquired insulin resistance, posing a short-term challenge to β-cells, with GDM developing in women whose β-cells are unable to cope (27). Previous research has suggested that the use of corticosteroids, such as betamethasone and dexamethasone, during pregnancy can lead to maternal hyperglycemia. This occurs because corticosteroids antagonize insulin synthesis and increase gluconeogenesis, resulting in elevated blood glucose levels. This effect is particularly concerning for pregnant women with diabetes, as it can further disrupt glucose homeostasis (11).
Although the placenta plays a role in protecting the fetus from the side effects of corticosteroids by extensively metabolizing them into inactive products (28), repeated use of corticosteroids can lead to reduced fetal body movements and breathing, potential intrauterine growth restriction, low birth weight, fetal hypoglycemia, and an increased risk of early-onset neonatal sepsis. Impaired glucose tolerance and reduced insulin sensitivity induced by corticosteroid therapy in a mother, even if transient, might be enough to affect fetal glucose hemostasis (29, 30). Overall, these findings underscore the importance of managing and monitoring blood glucose levels in pregnant women receiving corticosteroids to minimize disruptions in glucose metabolism and potential adverse effects on both maternal and neonatal health (14).
Collectively, these findings suggest that pregnant women with COVID-19 who also receive corticosteroids are at a higher risk of developing glucose intolerance. Although there is no available data on the number of people developing new hyperglycemia after COVID-19 and corticosteroid therapy, COVID-19 can induce new-onset diabetes in high-risk and susceptible populations and can worsen blood sugar control in those with preexisting diabetes (31). This aligns with our results, where the mean fasting blood sugar levels upon hospitalization were higher than 100 mg/dL, considered hyperglycemia during pregnancy, in women with COVID-19.
In a recent study comparing methylprednisolone and dexamethasone for the treatment of COVID-19 in non-pregnant individuals, hyperglycemia was more frequent in the methylprednisolone group, but it was managed without complications (32). Although this study did not report impaired glucose tolerance rates, our study found that approximately 40% of individuals developed glucose intolerance after receiving corticosteroids. However, all of them returned to normal glucose values within 4 weeks. Additionally, the rate of increased blood glucose was similar between the methylprednisolone and dexamethasone treatment groups.
The follow-up results for patients were also promising. The rates of preterm delivery and cesarean section, which are considered complications of COVID-19, were low and did not differ between treatment groups or patients with or without hyperglycemia.
This is the first study to evaluate the prevalence of transient hyperglycemia in pregnant women after corticosteroid therapy for COVID-19 infection. However, the current study had several limitations, including a small sample size in each group and limited follow-up data. Further studies with larger sample sizes and longer follow-up periods are required to assess the potential harmful and beneficial effects of different corticosteroid types in pregnant women with COVID-19 and their neonates.