1. Background
According to the World Health Organization (WHO) and the American College of Obstetricians and Gynecologists (ACOG), preterm labor (PTL) is defined as labor that begins before 37 weeks of pregnancy. Specifically, it occurs when regular contractions lead to the opening of the cervix between weeks 24 and 37 of gestation (1, 2).
Globally, premature birth affects 15 million infants annually, accounting for 12% of all deliveries. Approximately 50% of patients with threatened preterm labor will eventually experience preterm delivery (3, 4). Preterm labor is a significant risk factor for severe neonatal morbidities, including respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, sepsis, and cerebral palsy. It is also one of the leading causes of neonatal mortality (1, 5).
Several medications, such as beta-adrenergic receptor agonists, calcium channel blockers, nitric oxide-releasing drugs, magnesium sulfate, prostaglandin inhibitors, and oxytocin receptor antagonists, have been used to treat PTL. These agents are effective in delaying labor and improving neonatal and maternal outcomes (6-9). Calcium channel blockers like nifedipine and indomethacin have gained popularity as preferred tocolytic agents due to their lower incidence of side effects compared to beta agonists (10, 11).
However, side effects such as headaches, dizziness, flushing, and peripheral edema have been reported with nifedipine, and it is contraindicated in patients with low blood pressure, congestive heart failure, and aortic stenosis (12). Additionally, the concomitant use of nifedipine with magnesium sulfate, which is used to protect the baby's nerves during preterm labor treatment, is potentially dangerous (13).
Based on available scientific evidence, progesterone plays a crucial role in maintaining pregnancy by inhibiting uterine contractions (14). Animal studies have shown that progesterone reduces the concentration of oxytocin and alpha-adrenergic receptors in the myometrium, inhibits the local synthesis of prostaglandin F2α (PGF2α), and modulates the structure of the myometrium by preventing the formation of connections between myometrial cells, thereby reducing uterine contractions (15).
Recent studies have indicated that the use of progesterone during pregnancy is not associated with teratogenic complications and is effective in preventing PTL (16, 17). Various progestogens have been investigated to support endogenous progesterone in treating luteal phase deficiency. Dydrogesterone, a structural isomer of progesterone, is currently approved for clinical use during pregnancy to prevent miscarriage (9).
Dydrogesterone is an orally administered, high-affinity progesterone that binds almost exclusively to the progesterone receptor (18). It is a selective progesterone receptor agonist, similar in structure and pharmacology to endogenous progesterone, with higher oral bioavailability and a good safety profile. A daily dose of 20 mg Dydrogesterone is considered equivalent to 200 mg of vaginal progesterone (3, 19, 20).
Since 1960, approximately 113 million women and 20 million fetuses have been exposed to Dydrogesterone. Reported side effects are infrequent and include headaches, nausea, menstrual disorders, and weight gain. It is contraindicated in individuals with a known allergy to Dydrogesterone (21).
Long-term follow-up of fetuses exposed to Dydrogesterone has shown no differences in health status, physical examination, or neurophysiological development, as evaluated using the Ages and Stages Questionnaire (ASQ) (16, 17).
2. Objectives
To date, no evidence suggests that taking Dydrogesterone during pregnancy is harmful. The present study was conducted to determine the effectiveness of Dydrogesterone compared to nifedipine in preventing preterm labor.
3. Methods
3.1. Study Design and Participants
This was a randomized controlled clinical trial conducted on 54 pregnant women with single gestation, aged between 26 and 34 weeks of gestation, diagnosed with threatened preterm labor. The trial was carried out among women attending a teaching hospital (Hazrat Rasoul-Akram) in Tehran during 2016 - 2017. In this interventional study, preterm labor was defined as the occurrence of regular uterine contractions of ≥ 4 per 20 minutes or ≥ 8 per 60 minutes, with cervical dilation of ≥ 3 cm at a gestational age of less than 37 weeks. Threatened preterm labor was defined as the presence of regular uterine contractions without cervical changes. Uterine contractions were recorded using external tocodynamometry. The pressure-sensitive contraction transducer, called a tocodynamometer or TOCO for short, is a non-invasive and indirect measurement of intrauterine pressure that records the force exerted by the contracting abdomen during uterine contractions. (22) Cervical dilation and effacement were measured through vaginal examination. Baseline para-clinical data, including blood cell count and urine analysis, were collected. The gestational age of the women was determined based on the last menstrual period (LMP) and early pregnancy ultrasounds.
After case definition and based on our inclusion criteria, 54 women were randomly allocated to receive either oral Dydrogesterone (n = 27) or oral nifedipine (n = 27). The inclusion criteria were ages 18 to 45 years, single pregnancy, gestational age between 26 and 34 weeks, regular contractions, intact amniotic membrane, dilation less than 3 cm, and effacement less than 80%. Women with maternal or fetal conditions requiring immediate delivery (including fetal distress, chorioamnionitis, severe preeclampsia, placenta previa, or abruption), those entering the active labor phase, those who had vaginal bleeding, rupture of membranes, multiple gestation, polyhydramnios, any systemic infections, fever greater than 38°C, intrauterine growth restriction (IUGR), or blood pressure above 140/90 mm Hg, as well as pregnant women at risk for fetal abnormalities and cases with a history of thromboembolic events, were excluded from the trial.
We calculated the sample size of 27 in each group as follows:
3.2. Randomization and Intervention
The participants were randomly divided into the nifedipine and Dydrogesterone groups using a simple randomization method with a random number table and Excel software. Random allocation to each group was carried out by opening sealed, opaque envelopes indicating the assigned drug. Our intervention of interest was Dydrogesterone (Duphaston, ABBOT company) in comparison with nifedipine (Toliddaru Pharma company). After obtaining written informed consent, the intervention group received 40 mg of oral Dydrogesterone. Uterine contractions were monitored after 8 hours using a tonometer. If uterine contractions were reduced, the same drug was continued at a dose of 10 mg every 8 hours for 48 hours. If uterine contractions did not reduce after 8 hours of treatment with this drug, the patients were switched to nifedipine and excluded from the trial. The control group received oral nifedipine at a standard dose according to protocol, starting with a 10 mg dose, followed by administration every 15 minutes for an hour (maximum dose 40 mg in one hour), then 20 mg every 6 hours for 24 hours, and finally 20 mg every 8 hours for the next 24 hours.
3.3. Treatment Outcomes and Evaluation
Baseline characteristics of the two groups, including maternal age, gestational age, Body Mass Index (BMI), BISHOP score (determined by fetal station, cervical dilation, effacement, consistency, and position), uterine contractions (Montevideo units by tocodynamometry), gravidity, parity, history of preterm labor, history of cesarean section, cerclage, and infertility history, were measured and recorded in a checklist. The primary outcome of interest was the tocolytic efficacy of the two drugs, evaluated through uterine contraction. Other maternal and neonatal outcomes of interest included gestational age at delivery, type of delivery, interval between intervention and delivery, repeated preterm labor, BISHOP score, birth weight, admission to the neonatal intensive care unit (NICU), and duration of NICU stay.
All observations in both groups, including BISHOP score, uterine contractions, fetal heart rate, maternal heart rate, maternal blood pressure, and any maternal symptoms, were recorded on an observation sheet.
The study was designed as a double-blind trial; accordingly, the patients and the nurses who administered the drugs and evaluated the variables were blinded to the drug type and the trial objectives.
3.4. Ethical Considerations
This study was approved by the Ethics Committee of Iran University of Medical Sciences (Ethics code: IR.IUMS.REC1395.9211290013) and was also registered as a trial in the Iranian Registry of Clinical Trials (IRCT) (IRCT20180227038892N1). Before starting the trial, the purpose of the study was explained to all participants, and informed consent was obtained.
3.5. Statistical Analysis
Data analyses were conducted using SPSS software version 22 for Windows (SPSS Inc., Chicago, IL, USA). Qualitative data were evaluated using the chi-square or Fisher's exact test. The normal distribution of variables was assessed with the Kolmogorov-Smirnov test. Comparisons of quantitative parameters were performed using the independent sample t-test or the corresponding non-parametric test, the Mann–Whitney U test. A P-value of less than 0.05 was considered the threshold for statistical significance.
4. Results
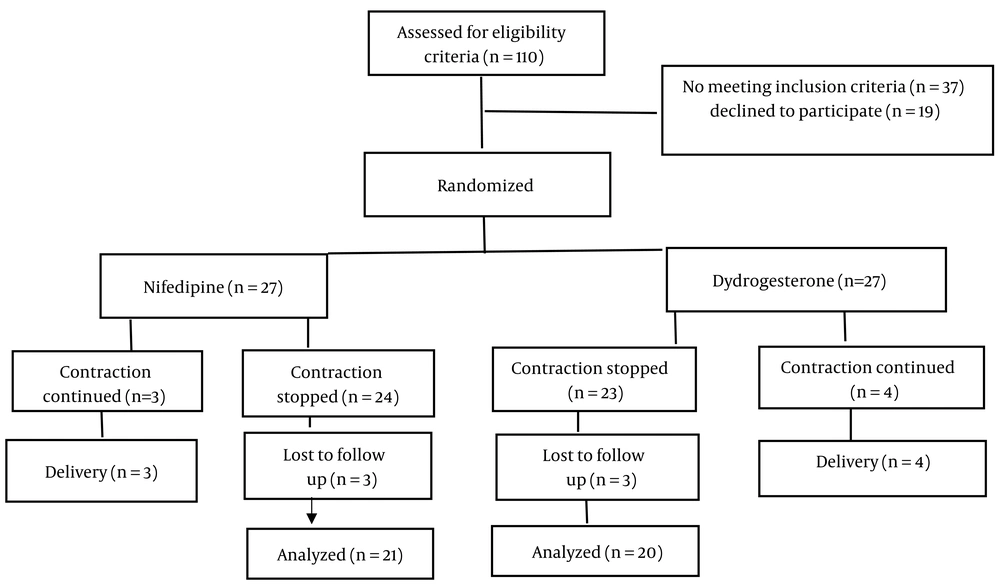
A total of 110 pregnant women were evaluated based on eligibility criteria, of which 54 (49%) were randomized 1: 1 to receive either oral Dydrogesterone (n = 27) or oral nifedipine (n = 27). Successful tocolysis was achieved in 85.2% of the women receiving Dydrogesterone and 88.9% of those receiving nifedipine. None of the women in the Dydrogesterone group progressed to the second-line protocol (nifedipine). Six women in the nifedipine group were excluded from the trial due to continued contractions (n = 3), preeclampsia (n = 1), and PPROM (n = 2). Similarly, seven women in the Dydrogesterone group were excluded due to continued contractions (n = 4), fetal distress (n = 1), and PPROM (n = 1). Ultimately, 20 women in the Dydrogesterone group and 21 women in the nifedipine group remained in the trial until the end of the follow-up and were analyzed (Figure 1).
There were no statistically significant differences between the two groups regarding baseline socio-demographic and clinical characteristics, such as age, gestational age, BMI, gravidity, parity, BISHOP score, uterine contractions, history of preterm birth, cesarean section, cerclage, and infertility (Table 1).
| Variables | Nifedipine (n = 27) | Dydrogesterone (n = 27) | P-Value |
|---|---|---|---|
| Age (y) | 27.78 ± 2.53 | 27.67 ± 2.53 | 0.8 |
| Gestational age (w) | 219.44 ± 11.89 | 218.56 ± 12.48 | 0.78 |
| BMI (kg/m2) | 25.67 ± 1.51 | 25.78+-1.42 | 0.76 |
| Bishop score | 1.3 ± 0.8 | 1.33 ± 0.8 | 0.9 |
| Uterine contraction (montevideo) | 91.94 ± 9.6 | 93.44 ± 11.3 | 0.7 |
| Gravid | 0.69 | ||
| 1 | 17 (63) | 17 (63) | |
| 2 | 8 (29.6) | 7 (25.9) | |
| 3 | 2 (7.4) | 2 (7.4) | |
| ≥ 4 | 0 (0) | 1 (3.7) | |
| History of preterm labor | 0.5 | ||
| Yes | 2 (7.4) | 1 (3.7) | |
| No | 25 (92.6) | 26 (96.3) | |
| History of cesarean section | 0.5 | ||
| Yes | 3 (11.1) | 2 (7.4) | |
| No | 24 (88.9) | 25 (92.6) |
a Values are expressed as mean ± SD or No. (%).
We followed the trial participants, 21 women in the nifedipine group and 20 women in the Dydrogesterone group, for maternal and neonatal outcomes until delivery. As shown in Table 2, the frequency of NICU admission in the Dydrogesterone group (15%) was lower than in the nifedipine group (23.8%), although this finding was not statistically significant (P = 0.4). Additionally, the mean NICU stay, another key neonatal outcome, was significantly lower in the Dydrogesterone group (2.3 days) compared to the nifedipine group (4.6 days) (P = 0.001).
| Variables | Nifedipin (n = 21) | Dyderogestrone (n = 20) | P-Value |
|---|---|---|---|
| Gestational age at the time of delivery (w) | 0.84 | ||
| Preterm | 8 (38.1) | 7 (35) | |
| Term | 13 (61.9) | 13 (65) | |
| Delivery type | 0.65 | ||
| NVD | 13 (61.9) | 11 (55) | |
| CS | 8 (38.1) | 9 (45) | |
| NICU admission | 0.42 | ||
| Yes | 5 (23.8) | 3 (15) | |
| No | 16 (76.2) | 17 (85) | |
| Interval between intervention and delivery (days) | 0.88 | ||
| 48 h | 3 (14.3) | 3 (15) | |
| One week | 3 (14.3) | 3 (15) | |
| More than one week | 15 (71.4) | 14 (70) | |
| Repeated preterm labor | 0.18 | ||
| Yes | 15 (71.4) | 11 (55) | |
| No | 6 (28.6) | 9 (45) | |
| Birth weight (gr) | 2780.9 ± 313.6 | 2767.5 ± 325.7 | 0.7 |
| NICU stay (days) | 4.6 ± 0.5 | 2.3± 0.5 | 0.001 |
| BISHOP score | 1.32 ± 0.8 | 1.35 ± 0.8 | 0.9 |
| Uterine contraction (montevideo) | 92.2 ± 9.6 | 94.44 ± 11.4 | 0.7 |
a Values are expressed as mean ± SD or No. (%).
We did not observe statistically significant differences between the two groups in terms of gestational age at delivery, type of delivery, interval between intervention and delivery, birth weight of neonates, BISHOP scores, or uterine contractions. Although not statistically significant, the frequency of repeated preterm labor, an important maternal outcome, was lower in the Dydrogesterone group (Table 2). Notably, no significant adverse effects were observed in either group.
5. Discussion
In our study, we found that the effects of nifedipine and Dydrogesterone were similar. The findings of this study highlight the fact that Dydrogesterone is effective in the treatment of preterm labor. Both drugs successfully prevented preterm labor. While nifedipine is one of the standard drugs used to prevent preterm labor, it has side effects and may not be suitable for some patients.
According to the literature, various drugs with different administration methods have been used to treat preterm labor. Several studies have compared the effects of these drugs and their methods of administration, but there is no strong evidence supporting the superiority of any one drug for the treatment of preterm labor. The inconsistency and, at times, contradictory results reported in previous studies demonstrate the need for further research, particularly randomized clinical trials, to obtain strong evidence about the most effective treatment in this field. (1, 2, 9, 12)
Numerous studies have shown the effectiveness of nifedipine as a key drug for the treatment of preterm labor, and it is now used as a first-line tocolytic therapy (1, 10, 12). In a systematic review and meta-analysis conducted by Conde-Agudelo et al., nifedipine was reported to be superior to β2-adrenergic receptor agonists in the treatment of preterm labor. Similarly, in a quasi-experimental study (2020) by Habib et al., nifedipine was shown to be effective in inhibiting uterine contractions and delaying labor for more than 48 hours (23).
Although El-Sayed et al. (2021) demonstrated that combining oral nifedipine with vaginal progesterone led to a more rapid response to tocolysis in threatened preterm labor compared to oral nifedipine alone, no evidence has been found on the use of Dydrogesterone as the primary drug for the treatment of preterm labor, based on a review of the available scientific literature. This trial is one of the few studies investigating the effectiveness of Dydrogesterone in the treatment of preterm labor (9).
Several experimental studies have evaluated the effect of Dydrogesterone in this context. Hudic, I et al. reported that the use of Dydrogesterone in women at risk of preterm delivery increased the production of progesterone-induced blocking factor (PIBE) and interleukin-10, while decreasing interferon-gamma levels. According to their findings, Dydrogesterone could be effective in preventing or treating preterm labor (24). Conversely, a study conducted in 2016 on 48 pregnant women by Wilasinee Areeruk and Vorapong Phupong in Thailand found that adjuvant treatment with oral Dydrogesterone (20 mg/day), compared to placebo, did not reduce recurrent uterine contractions or prolong the latency period (3).
Even though the present study supports the use of Dydrogesterone, the conflicting results from previous studies prevent us from drawing a definitive conclusion about its effectiveness. Some studies, such as those by Haghighi et al. (25) and Lotfalizadeh et al. (11), align with our findings and emphasize the effectiveness of Dydrogesterone. However, other researchers, like Thongchan et al. (26), have been unable to demonstrate its efficacy.
5.1. Conclusions
The present study offers several potential benefits. One of the most notable advantages is that it is among the few studies evaluating the effectiveness of Dydrogesterone in preventing preterm labor. Another key benefit is the comparison of Dydrogesterone's effectiveness to the existing standard treatment, nifedipine. However, the small sample size is the major limitation of this study. The findings suggest that Dydrogesterone is effective in treating preterm labor. Therefore, conducting further studies with a larger sample size is essential to either confirm or refute this result. Our trial demonstrated that both Dydrogesterone and nifedipine have approximately the same effect on preterm labor. Additionally, we found that Dydrogesterone could be a useful alternative, particularly when the use of nifedipine is limited or contraindicated.