1. Context
Endometrial-like tissue is present outside the uterus in endometriosis, a complex gynaecological illness that causes severe discomfort and inflammation (1, 2). While the exact cause of endometriosis is still unknown, elements such as immune system malfunction and retrograde menstruation may contribute to its development (1). This review aims to explore the interplay between psychological stress and the manifestation of endometriosis symptoms, specifically focusing on how stress might influence pain perception and inflammatory processes in affected individuals. Understanding this relationship is crucial, as it could reveal new insights into the management and treatment of endometriosis, potentially leading to improved therapeutic strategies and an enhanced quality of life for patients.
Recent advances in research have uncovered a number of important components of the condition, which add to its complex nature. These include genetic predisposition, the function of the immune system, and hormonal imbalances. Research has brought attention to the role that neuroimmune interactions play in the development of endometriosis, specifically the ways in which immune cells and inflammatory pathways affect pain mechanisms (3). Additionally, new pharmaceutical approaches, including hormone modulators and immunotherapies, have shown promise in symptom management, and advances in imaging techniques and biomarkers have improved diagnostic accuracy (4).
Though more recent research has focused on the role of epigenetic modifications and the systemic inflammatory response in the development of endometriosis, seminal studies such as those on the theory of retrograde menstruation and the involvement of the peritoneal immunological milieu remain foundational (5). In addition, new clinical studies have assessed the effectiveness of stress-reduction methods, such as mindfulness and cognitive-behavioural therapy (CBT), in managing pain perception in women affected by endometriosis.
Notwithstanding these developments, there remains a significant knowledge gap regarding the interaction among psychological stress, pain perception, and inflammatory processes in endometriosis. By investigating the possible mechanisms through which psychological stress may influence these variables, this review seeks to address this gap. By clarifying this connection, we aim to expand our understanding of endometriosis and offer new treatment approaches that address both the psychological and physical manifestations of the condition, thereby enhancing the lives of those affected.
2. Evidence Acquisition
A thorough examination of the current literature was conducted to comprehensively explore the relationship between psychological stress, pain perception, and inflammatory responses in women with endometriosis. We performed an extensive search using well-known academic databases such as PubMed, Scopus, and Google Scholar. Our aim was to collect a wide range of papers and reviews that investigate the physiological and psychological aspects of pain and inflammation associated with endometriosis. The literature search sought to identify crucial studies that offer a comprehensive analysis of the impact of stress on these factors and its potential role in exacerbating endometriosis symptoms. Through data analysis, our objective was to clarify the mechanisms by which stress influences pain and inflammation, as well as to identify potential pathways linking psychological stress with the clinical symptoms of endometriosis. This comprehensive body of evidence enabled a detailed understanding of the potential effects of stress on disease progression and symptom severity, thereby laying a foundation for future research and improvements in therapeutic practices.
3. Results
3.1. Endometriosis
The exact cause of the intriguing condition known as endometriosis is still unknown (1, 2). Congenital, environmental, epigenetic, autoimmune, and allergic factors are among the proposed etiological contributors. The primary process thought to be responsible for the development of endometriosis lesions is retrograde menstruation, which is defined as the movement of menstrual blood through the fallopian tubes into the peritoneal cavity and the subsequent implantation of shed endometrial cells (6).
The presence of endometrial glands and stroma, resembling lesions outside the uterus, is a characteristic feature of endometriosis (1, 7). These lesions can manifest as ovarian cysts, superficial implants, peritoneal lesions, and deep infiltrating disease. Although the exact cause of endometriosis remains unknown, several theories exist regarding the formation of endometriotic lesions. In both humans and non-human primates, retrograde menstruation is a potential process that occurs during the menstrual cycle (8). It involves the movement of the endometrial lining from the uterus through the fallopian tubes into the pelvic cavity. The reverse flow and the potential for endometrial cells to spread via the lymphatic or circulatory systems may lead to the implantation of endometrial tissue in ectopic locations. While retrograde menstruation is common (possibly universal among menstruating women), endometriosis is significantly less prevalent (9, 10).
Endometriosis is a gynaecological disease that typically affects the ovaries and peritoneum. It is characterized by the formation of tissue resembling the endometrium outside the uterus. This condition results in premenstrual pain and painful menstrual cycles. The most widely accepted explanation for endometriosis is that retrograde menstruation implants endometrial tissue in the peritoneal cavity. This original theory suggests that endometriosis develops when waste products and shed endometrial cells travel backward through the fallopian tubes into the pelvic cavity during menstruation (3, 7). Retrograde menstruation occurs in a large percentage of women (76% to 90%) with patent fallopian tubes (3, 11). It is important to note, however, that not all of these women develop endometriosis. The condition was first described by Daniel Shroen in his work Disputatio Inauguralis Medica de Ulceribus Ulceri in 1690 (6).
3.1.1. Symptoms of Endometriosis
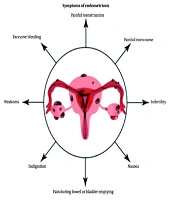
Pelvic discomfort is the primary manifestation of endometriosis and is typically linked to the menstrual cycle. While it is common for individuals to experience cramps during menstruation, women with endometriosis often endure significantly more severe menstrual pain than the average person. Furthermore, the discomfort may intensify over time. The risk factors for endometriosis include genetic predisposition, immune system disorders, hormonal imbalances, abdominal surgery, and age (12). Figure 1 illustrates some of the symptoms.
Symptoms of endometriosis (3); this figure shows the symptoms associated with endometriosis.
3.1.2. Pathophysiology of Endometriosis
Endometriosis is an intricate and multifaceted illness with an aetiology that has not yet been fully discovered. Various factors have been closely associated with the development of the condition, although a definitive aetiology remains undetermined (12). Sampson's theory of retrograde menstruation is commonly considered the leading explanation for the development of ectopic endometriotic tissue (6, 13). According to this theory, endometrial cells present in abnormal locations do not originate there; rather, they travel through the fallopian tubes into the peritoneal cavity during the menstrual cycle. Three key conditions must be met for endometriosis to occur: Retrograde menstruation, the presence of viable endometrial cells in the menstrual outflow, and the successful adhesion and continued proliferation of these cells on the peritoneum following implantation. This theory has faced considerable criticism. Despite the fact that endometriosis is relatively uncommon in the general population, nearly 90% of menstruating women experience retrograde menstruation (13-15). Studies have discovered no significant difference in the presence of endometrial cells in the peritoneal fluid (PF) of individuals with and without endometriosis (16, 17).
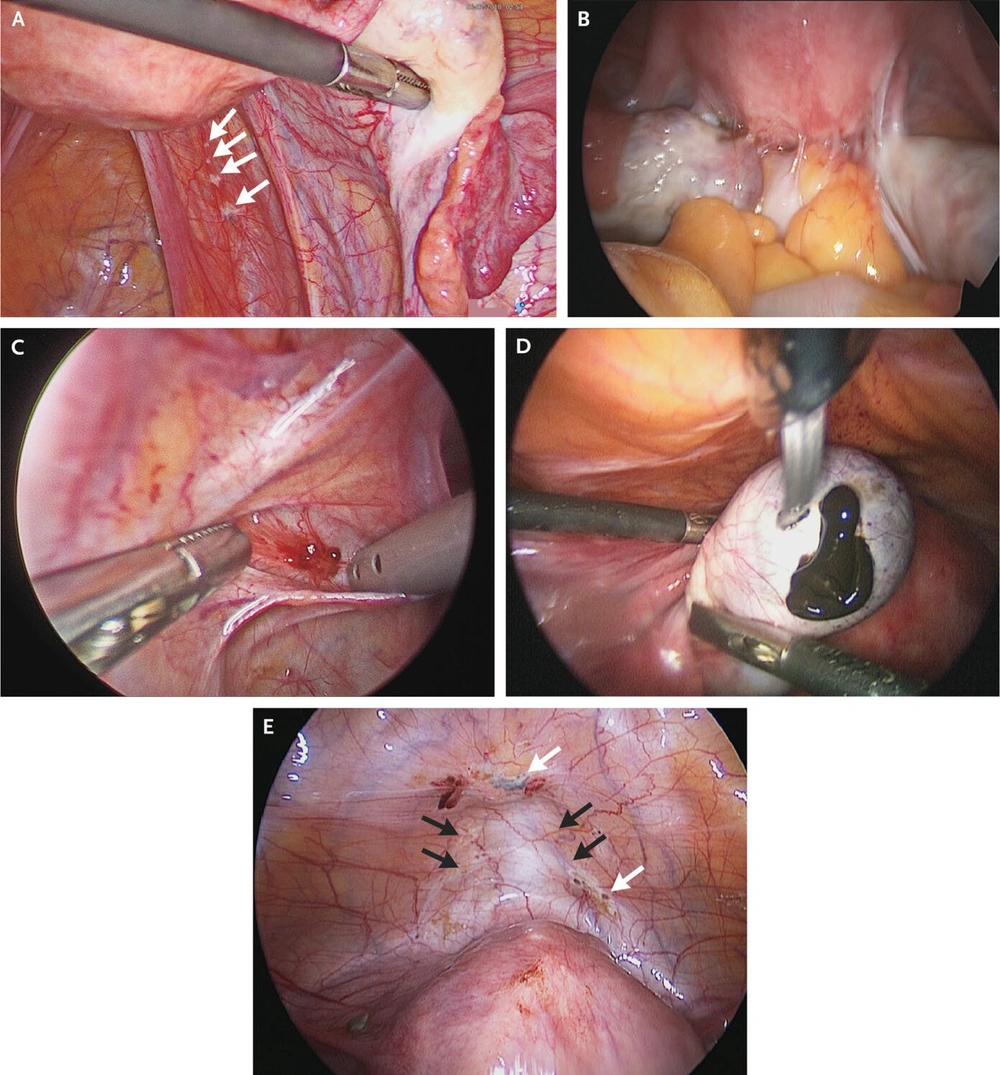
However, one could argue that the mere presence of endometriotic tissue in the PF of healthy individuals is insufficient to invalidate Sampson's theory. Another study identified a significant correlation between the number of endometrial cells in the PF and the severity of the disease (2, 9). This variation may be associated with the volume and frequency of retrograde menstrual flow, as women with endometriosis typically experience shorter menstrual cycles and heavier bleeding, leading to a greater volume of endometrial material in the pelvic cavity (14). Nevertheless, the theory of retrograde menstruation cannot account for cases of endometriosis in girls prior to menarche, in female foetuses, and even in males (16). Table 1 and Figure 2 illustrate the different presentations of endometriosis.
| Stages | Description |
|---|---|
| One | This minor illness is characterized by isolated implants. Adhesions are not present. |
| Two | This is a modest type of the disease where superficial implants cover the ovaries and peritoneum. None of the adhesions were noticeable. |
| Three | The contemporary condition is characterized by several implants, both highly intrusive and superficial. It is possible to develop adhesions on the fallopian tubes and ovaries. |
| Four | Multiple deep and superficial implants, enormous ovarian endometriomas, and other symptoms are indicative of severe illness. Dense adhesions are usually found. |
a This table outlines the four stages of endometriosis, ranging from minimal to severe, based on the extent of endometrial tissue growth and the presence of adhesions.
Stages of endometriosis (18); A, minimal endometriosis with four peritoneal endometriotic lesions (white arrows) on the right pelvic side wall; B, extensive endometriosis with bowel adhesions to the uterus; C, superficial red peritoneal endometriotic lesion and hyperemia; D, endometrioma (chocolate cyst) in the left ovary; E, deep bladder nodule (black arrows) and red, brown, and black peritoneal endometriotic lesions (white arrows).
3.1.3. Types of Endometriosis
There are three different types of ectopic endometrial lesions, which include endometrial glands and stroma, classified based on their location and physiopathology (19).
3.1.3.1. Peritoneal Endometriosis
The term peritoneal endometriosis refers to the presence of endometrial tissue on the outer layer of the peritoneum. Ovarian endometriosis occurs when endometrial tissue is located within the ovaries. Deep infiltrating endometriosis describes endometrial tissue that has deeply penetrated surrounding structures. The condition can present in various forms, including white infiltrations on the peritoneum, peritoneal surface irregularities, red, brown, black-blue, and black lesions, translucent vesicles lacking color, and localized swollen blood vessels. Peritoneal endometriosis is observed in approximately 15 - 50% of women diagnosed with endometriosis. However, diagnosing peritoneal endometriosis remains challenging due to its poor visibility on imaging studies and the need for laparoscopy to achieve a definitive diagnosis (18, 20).
3.1.3.2. Ovarian Endometrioma
Ovarian endometrioma (OMA) refers to cysts within the ovaries that are lined with endometrial epithelium and filled with thick, dark fluid (19). The condition affects between 2% and 10% of women of reproductive age and is found in 50% of women undergoing infertility treatment (19).
3.1.3.3. Deep Infiltrating Endometriosis
Deep infiltrating endometriosis (DIE) is characterized by a specific histologic pattern that may include well-developed glandular cells, pure stromal cells, glandular or mixed differentiated cells, and pure undifferentiated glandular cells (19). Although the overall mortality rate associated with endometriosis is generally low, DIE can significantly affect health outcomes. Studies estimate that the prevalence of DIE is approximately 2% among women of reproductive age. While mortality directly caused by endometriosis remains rare, complications related to DIE — such as bowel obstruction or infertility — can contribute to increased health risks (19).
3.1.4. Diagnosis of Endometriosis
Endometriosis is a prevalent condition; however, its diagnosis can often be delayed for 8 to 12 years due to the wide variety of symptoms it presents. Currently, laparoscopy is considered the most accurate diagnostic technique. Other diagnostic methods include magnetic resonance imaging (MRI), ultrasound, and a thorough history and physical examination (21).
3.2. Stress and Its Potential Impact on Health
Stress is a cognitive response to a perceived threat or danger. It can be either transient or chronic and can impact hormones, mood, susceptibility to illness, and overall well-being. The physiological consequences of stress include fatigue, depression and anxiety, migraines, insomnia or disturbed sleep patterns, cardiovascular complications such as myocardial infarction, and impaired immune system function (22, 23).
Chronic stress can have long-term effects on both the body and the brain. Prolonged or persistent stress may weaken the immune system, increasing vulnerability to a range of illnesses — from common colds to more serious health conditions. During stressful situations, the body releases a hormone known as cortisol, which circulates throughout the bloodstream. Cortisol plays a crucial role in regulating several physiological processes, including sleep, weight, blood pressure, and blood glucose levels.
However, when cortisol levels remain elevated due to prolonged stress, it can lead to chronic inflammation and a weakened immune system, partly due to a reduction in white blood cell count (24, 25). A meta-analysis of multiple studies investigating the relationship between psychological stress and endometriosis symptoms found that women experiencing high stress levels reported a significant increase in pain severity compared to those with lower stress levels. For instance, another study found that women with high stress reported a 40% increase in pain intensity compared to those with low stress. These findings underscore the importance of addressing psychological stress as part of a comprehensive strategy for managing endometriosis.
3.3. Physiological Mechanisms of Pain
The International Association for the Study of Pain (IASP) has provided the most widely recognized and current definition of pain as an unpleasant emotional and sensory experience that is associated with, or resembles that associated with, actual or potential damage to bodily tissues. Despite the existence of several theoretical models proposed to explain the physiological basis of pain, none has been able to fully encapsulate all aspects of the pain experience.
The four primary theories of pain perception are the specificity theory, intensity theory, pattern theory, and gate control theory. However, in 1968, Melzack and Casey proposed that pain should be understood as a multidimensional experience, consisting of components that are interrelated rather than independent. These dimensions include the affective-motivational, sensory-discriminative, and cognitive-evaluative components of pain (26, 27).
3.3.1. Pain Mechanisms
3.3.1.1. Nociceptive Pain Mechanism
Nociception refers to the pathological process that occurs in peripheral organs and tissues. It involves the perception of pain projected into a damaged body part or experienced in a different location, known as referred pain. Nociceptors in the target tissue can be activated by triggers related to temperature, mechanical stimuli (such as stretching or straining), and chemical stimuli (such as pH changes caused by local inflammation). As a result, noxious stimuli may fall into one of these three categories. Nociceptive pain occurs when harmful chemical (inflammatory), mechanical, or ischaemic stimuli activate the peripheral receptive terminals of primary afferent neurons (28, 29).
3.3.1.2. Neuropathic Pain Mechanism
The IASP (2011) defines neuropathic pain as pain resulting from a disease or injury affecting the somatosensory nervous system. This type of pain can result from damage to any part of the neuroaxis of the peripheral nervous system or to the spinal or supraspinal nervous system. Neuropathic pain is categorized into two main types: (1) The term “central neuropathic pain” refers to pain caused by a specific disease or injury that affects the central somatosensory nervous system; (2) peripheral neuropathic pain is defined as pain resulting from disease or injury to the peripheral somatosensory nervous system (28).
3.3.1.3. Nociplastic Pain Mechanism
According to the IASP (2011), nociplastic pain arises from altered nociception, despite the absence of clear evidence of actual or threatened tissue damage or disease or lesion of the somatosensory system.
3.3.1.4. Central Sensitization
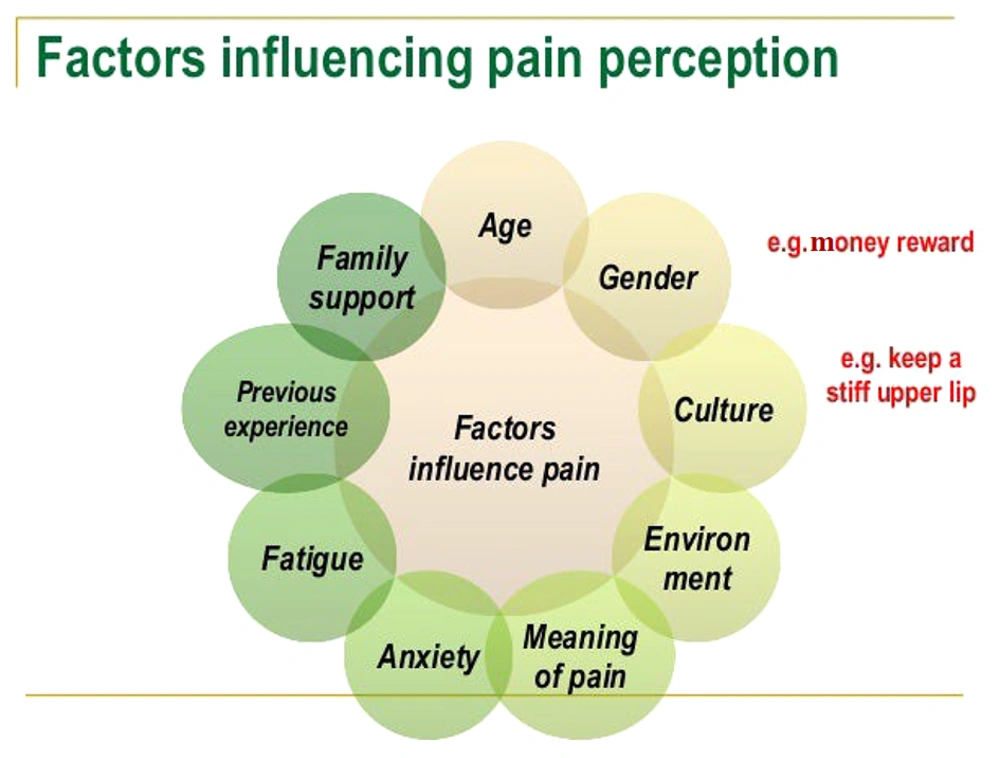
Central sensitization, as defined by the IASP (2011), is an increased responsiveness of nociceptive neurons in the central nervous system to normal or sub-threshold afferent input, leading to hypersensitivity to stimuli. This form of pain often does not respond well to standard pharmacological treatments. Therefore, a personalized treatment strategy is typically required, addressing factors such as lifestyle, mood, physical activity, employment, and social circumstances (27). Figure 3 illustrates several factors that influence pain perception.
Factors affecting pain perception (22)
3.4. Physiological Effects of Stress
Stress significantly impacts various physiological systems, including the nervous, muscular, reproductive, endocrine, digestive, respiratory, and circulatory systems (30). The endocrine system plays a central role by producing steroid hormones — such as cortisol — that initiate the stress response (30). Stress activates the sympathetic nervous system (SNS), which stimulates the adrenal glands to release catecholamines. Once the acute stress episode is resolved, the parasympathetic nervous system (paraSNS) aids in the body’s recovery (30).
Acute stress results in an increased heart rate, stronger cardiac muscle contractions, temporary heart enlargement, and redirection of blood flow to major muscle groups (31). The circulatory and respiratory systems collaborate to remove carbon dioxide and deliver oxygen to tissues. However, when acute or chronic stress leads to dysregulation of the autonomic nervous system (ANS), it can trigger inflammation and bronchial hyperresponsiveness (32). This dysregulation may alter breathing patterns, resulting in symptoms such as tachypnea and dyspnoea, which can exacerbate existing respiratory issues.
Catecholamines like norepinephrine and adrenaline — released during stress — also influence the digestive tract. These hormones interact with adrenergic receptors throughout the gastrointestinal system. Specifically, activation of α-adrenergic receptors in intestinal smooth muscle reduces intestinal motility and delays gastric emptying (31).
Prolonged stress activates a cascade of physiological responses, including elevated secretion of catecholamines and cortisol, which impact the musculoskeletal system (32). Chronic exposure to high cortisol levels enhances osteoclast activity while inhibiting osteoblast function, potentially leading to decreased bone density and muscle atrophy.
When a stressor is detected, the body alerts the brain, which then signals the hypothalamus to activate the ANS. The sympathetic response results in increased muscle strength, elevated heart rate and blood pressure, increased levels of circulating fats and sugars, delayed blood clotting, reduced bowel activity, and a systemic hormonal response (32).
The adrenal glands play a pivotal role in the body’s stress response alongside the nervous system. Located atop each kidney, the adrenal glands — particularly the adrenal medulla — are directly connected to the SNS via neuronal pathways. Upon stimulation by the SNS, the adrenal medulla releases adrenaline and noradrenaline into the bloodstream. This initiates the "fight or flight" response, characterized by an elevated metabolic rate for energy mobilization, bronchodilation for improved respiration, and an increased heart rate (33).
3.4.1. Impact of Chronic Stress on the Body
Persistent stress, defined as ongoing stress experienced over an extended period, can contribute to long-term complications in the cardiovascular system. A continuous and progressive elevation in heart rate, along with increased levels of stress hormones and blood pressure, can negatively affect the body. Chronic and persistent stress significantly increases the risk of developing hypertension, heart attacks, and strokes (30).
Chronic stress is also associated with inflammation within the circulatory system, particularly in the coronary arteries. This inflammatory response is believed to be one of the mechanisms linking stress to myocardial infarction. Additionally, an individual’s physiological reaction to stress may influence their cholesterol levels, further contributing to cardiovascular risk. The relationship between stress and cardiovascular disease appears to differ among women based on their menopausal status — whether premenopausal or postmenopausal (30). Premenopausal women typically benefit from higher estrogen levels, which improve the ability of blood vessels to respond adaptively to stress. This physiological resilience supports more effective stress regulation and offers protection against heart disease (30).
In contrast, postmenopausal women experience a decline in estrogen levels, resulting in the loss of this protective cardiovascular effect. As a result, they are more susceptible to the detrimental influence of stress on heart health (16, 30).
3.4.2. Mechanisms by Which Stress Might Influence Pain Perception
3.4.2.1. Psychological Modulation
Stress can alter cognitive processing and attentional focus, leading to increased sensitivity to pain stimuli. It may also heighten the likelihood of perceiving pain as more intense than it actually is (34).
3.4.2.2. Physiological Response
Stress activates the SNS, resulting in elevated blood pressure and heart rate. This physiological arousal can amplify pain signals and lower the threshold for pain perception, making individuals more susceptible to experiencing pain (35).
3.4.2.3. Inflammatory Pathways
Stress may exacerbate pain by sensitizing nerve endings and enhancing pain perception through the body's inflammatory responses (36).
3.4.2.4. Endogenous Opioids
Stress can influence the release and regulation of endogenous opioids — naturally occurring, pain-relieving substances produced by the body. Alterations in their activity can affect both pain sensitivity and overall pain perception (37).
3.4.2.5. Alterations in the Central Nervous System
Chronic stress can lead to anatomical and functional changes in the brain, particularly in regions associated with pain processing and regulation, such as the amygdala and prefrontal cortex (38).
3.4.3. The Mind-Body Connection in Endometriosis
The occurrence of recurring and persistent pain, combined with inadequate recognition by healthcare providers, can result in various psychological and interpersonal difficulties. These challenges can be as distressing for the patient and her partner as the physical pain itself (39). Chronic stress disrupts the body’s hormonal balance, particularly affecting the production and regulation of hormones involved in the reproductive system. Elevated levels of cortisol — a primary stress hormone — can interfere with the normal functioning of the hypothalamus-pituitary-gonadal (HPG) axis, which governs the production of estrogen and progesterone. This hormonal imbalance may contribute to irregular menstrual cycles, altered ovulation, and other symptoms associated with endometriosis.
A considerable number of patients report emotional responses such as shame, feelings of inadequacy, low self-esteem, and negative body image. Women with persistent genital discomfort demonstrate higher levels of both state and trait anxiety, as well as increased depression scores, when compared to healthy women. Additionally, they frequently exhibit elevated levels of catastrophizing — characterised by exaggerated, negative perceptions of pain, including magnification, rumination, and helplessness. These women also display increased hypervigilance towards pain, heightened fear of pain, and lower self-efficacy, which refers to their belief in their ability to manage pain effectively. These findings stand in contrast to the experiences of women without chronic pain (39).
Women diagnosed with endometriosis also experience significant psychological distress, often manifesting as guilt toward their partner, a diminished sense of femininity, altered body image, a loss of bodily autonomy, feelings of worthlessness, hopelessness, and difficulties in identifying and expressing emotions — known as alexithymia. Compared to healthy individuals, these women display significantly higher levels of pain catastrophising, stress, depression, and anxiety (39).
Stress can profoundly influence the proliferation and spread of endometrial tissue beyond the uterus, exacerbating key symptoms of endometriosis such as pain and inflammation. Chronic stress activates the immune system to produce inflammatory responses, which not only encourage the growth of ectopic endometrial tissue but also intensify the associated pain and suffering. Furthermore, stress can lead to increased muscle tension and spasms, thereby aggravating pelvic discomfort. It may also disrupt hormonal balance, contributing to menstrual irregularities — a common symptom in individuals with endometriosis (40). The multifaceted impact of stress on the physical and psychological dimensions of endometriosis underscores the critical importance of stress management in mitigating symptoms and improving the overall well-being of those affected by the condition (40).
3.5. Inflammation and Endometriosis
Inflammation plays a critical role in the development and persistence of ectopic endometrial tissue in endometriosis. When endometrial cells migrate beyond the uterus, the body’s immune system is activated, resulting in an inflammatory response. This response serves not only as a defense mechanism but also actively contributes to the attachment of endometrial cells to pelvic organs such as the ovaries, fallopian tubes, and peritoneum. The immunological reaction creates an inflammatory environment that supports the adhesion, survival, and proliferation of these ectopic cells (40, 41).
Once these cells adhere to surrounding tissues, they initiate the formation of endometriotic lesions, which themselves produce inflammatory mediators. This process creates a self-perpetuating cycle: Inflammation promotes the growth and maintenance of endometriotic tissue, and the lesions, in turn, generate further inflammation. As a result, persistent inflammation disrupts normal tissue function, giving rise to symptoms such as chronic pelvic pain, dysmenorrhea (painful menstruation), and infertility. Ongoing inflammation can lead to the formation of fibrosis and adhesions (42). Fibrosis refers to the excessive development of fibrous connective tissue, which can cause organs to adhere to one another, leading to severe pain and impaired function of the affected organs (43). Adhesions are bands of scar tissue that bind organs together, further exacerbating the clinical symptoms of endometriosis and interfering with the normal operation of the reproductive system. Endometriosis is thus characterized as a chronic and often debilitating condition, with inflammation at the core of its pathophysiology. The continuous inflammatory process significantly diminishes the quality of life for those affected by this disorder (43, 44).
3.5.1. Inflammatory Markers Commonly Measured in Endometriosis
Inflammatory markers play a vital role in the diagnosis and monitoring of endometriosis, providing valuable insights into the presence and severity of the condition.
3.5.1.1. Blood-Based Markers
The CA125, a glycoprotein often found at elevated levels in the bloodstream of women with endometriosis, is the most frequently suggested biomarker. Although increased CA125 levels may indicate the presence of endometriotic tissue, it is important to recognize that this marker is not specific to endometriosis. Elevated levels can also be observed in other conditions, such as ovarian cancer and pelvic inflammatory disease. Therefore, while CA125 is useful for raising clinical suspicion and monitoring the progression of endometriosis, it should always be interpreted in conjunction with other diagnostic modalities (45).
Other markers, such as CA19-9 and CA72-4, have been studied for their increased specificity in differentiating endometriosis from other gynaecological disorders. These markers may be particularly helpful in cases where CA125 values are inconclusive, providing additional evidence to support the diagnosis of endometriosis.
Recent studies have also highlighted the potential of circulating microRNAs (miRNAs) as novel biomarkers. These small, non-coding RNA molecules offer insights into the role of endometrial stem cells in the pathogenesis of endometriosis, potentially contributing to a deeper understanding of the molecular mechanisms underlying the disease. Detecting specific miRNAs in the bloodstream may signal the presence and activity of endometriotic lesions, offering a promising avenue for early and more precise diagnosis (46).
3.5.1.2. Urine-Based Markers
Emerging research is also exploring urine-based biomarkers as a non-invasive method for early detection and screening of endometriosis. These urinary markers hold the potential to revolutionize the diagnostic process by offering a less invasive and more accessible alternative to traditional techniques. Early and accurate identification of the disease is critical for effective management and treatment. Urine-based diagnostics could significantly reduce the reliance on invasive procedures such as laparoscopy, which is currently regarded as the gold standard for diagnosis. By enabling earlier detection, these markers may facilitate timely intervention, thereby improving clinical outcomes and enhancing the quality of life for individuals affected by endometriosis (47).
3.5.2. Potential Link Between Stress and Inflammatory Response in Endometriosis
The correlation between chronic stress and endometriosis highlights the intricate interplay between psychological well-being and physical health, demonstrating how stress can significantly influence the pathophysiology of this disorder. Persistent stressors activate the hypothalamic-pituitary-adrenal (HPA) axis, a central component of the body’s stress response system (40). This activation leads to the prolonged release of cortisol and other stress-related hormones, which are essential for managing acute stress but may become harmful when elevated over extended periods (40).
Under chronic stress conditions, persistently elevated cortisol levels can have multiple detrimental effects on the immune system (48). At high concentrations, cortisol acts as an immunosuppressant (48). This suppression reduces the body’s ability to mount effective immune responses to pathological processes, including the inflammation characteristic of endometriosis (49). A weakened immune system impairs the body’s capacity to control the spread of endometrial cells beyond the uterus (33), thereby facilitating the adhesion, survival, and proliferation of ectopic tissue and intensifying inflammation (33). In addition to its physiological effects, chronic stress also profoundly affects psychological and emotional health. Sustained stress often leads to increased emotional distress, which can heighten the perception of pain and other symptoms associated with endometriosis. This creates a vicious cycle, where intensified pain exacerbates psychological stress, further amplifying the subjective experience of illness (48).
Findings from research on other chronic conditions — such as cancer, inflammatory bowel disease, and multiple sclerosis — consistently show that persistent stress can worsen disease progression and symptom severity. These conditions offer supporting evidence that stress accelerates the deterioration of chronic illnesses via mechanisms involving inflammation and immune suppression, mechanisms that are equally relevant to endometriosis. Understanding the pivotal role of stress in the context of endometriosis underlines the importance of a comprehensive, integrative treatment approach (50). Incorporating psychological interventions alongside standard medical treatments has the potential to improve health outcomes. Stress management strategies such as CBT, mindfulness, and relaxation techniques can help reduce emotional distress and may also modulate inflammatory responses. This integrated approach could significantly enhance the overall management of endometriosis, improving both psychological well-being and physical symptoms (50).
3.6. Future Direction
Future research should prioritize the development and evaluation of integrated treatment strategies for endometriosis. Combining psychological interventions, such as CBT, with conventional pharmaceutical treatments may offer a more holistic and effective approach to disease management. This integrative model acknowledges both the physiological and psychological dimensions of endometriosis, potentially enhancing symptom control and overall patient well-being. Additionally, the identification of biomarkers associated with stress responses in individuals with endometriosis could provide critical insights into the disorder’s underlying mechanisms. Such biomarkers may facilitate the implementation of personalized treatment strategies, allowing clinicians to tailor interventions based on individual physiological and psychological profiles. Advancements in these areas of research have the potential to significantly improve clinical outcomes and quality of life for those affected by endometriosis, underscoring the importance of a multidisciplinary approach to both care and investigation.
4. Conclusions
Endometriosis is a complex gynaecological disorder characterized by the ectopic growth of endometrial-like tissue outside the uterus, leading to substantial pain and inflammation. Although the precise aetiology remains unclear, prevailing theories suggest contributions from retrograde menstruation, immune dysfunction, and other systemic factors. This review has explored the role of psychological stress in modulating the symptoms of endometriosis, with particular emphasis on pain perception and inflammatory responses. Current evidence indicates that stress may exacerbate endometriosis by amplifying inflammatory activity, altering pain sensitivity, and disrupting hormonal balance. Chronic stress triggers the sustained release of cortisol, which can suppress immune function and enhance pain perception through both inflammatory pathways and neuroendocrine mechanisms. Integrating stress management techniques — such as cognitive-behavioral therapy, mindfulness, and relaxation strategies — assinto standard treatment protocols holds promise for improving therapeutic outcomes. These approaches may not only alleviate psychological distress but also reduce physiological symptom burden, ultimately enhancing the quality of life for individuals affected by this debilitating condition.