1. Background
Cardiovascular diseases (CVDs) are major causes of disability and premature death in the world, and they substantially increase health care costs, mortality, and morbidity (1). Numerous cellular components have been suggested and investigated in CVDs, including cytosolic redox changes, mitochondrial disorders, inflammatory-immune factors, adhesion molecules, and apoptosis (2). Apoptosis is one of the most important causes of CVDs, especially heart failure, due to the limited capability of cardiac cells against injury and regeneration (3-5). In this regard, Whelan et al. (6) reported that the rate of apoptosis in the normal myocardium is very low as 0.001% - 0.002%. However, only a slight increase of 0.023% in apoptosis caused by increased expression of caspase-8 in cardiac cells of mice can result in dilated cardiomyopathy (7).
Apoptosis occurs through both intrinsic and extrinsic pathways. The extrinsic pathway is triggered by the binding of key ligands, including TNFα and Fas to death-inducing membrane receptors. In contrast, the intrinsic pathway, the most important pathway for apoptosis, is accompanied by changes in mitochondrial permeability and the release of apoptotic factors. The molecular events of apoptosis are principally determined by the balance between pre- and anti-apoptotic regulatory proteins. In this process, Bax and Bcl2 are the key proteins involved as the initiators of mitochondrial apoptosis (4, 8). Evidence shows that the Bax protein can decrease the stability of the mitochondrial outer membrane, pierce it, and result in the release of apoptotic factors such as cytochrome C. In contrast, the Bcl2 protein inhibits the pre-apoptotic activity of Bax protein and maintains the mitochondrial membrane integrity (4, 8). Hence, all apoptotic pathways eventually lead to the activation of caspase-3 and degradation of vital cell proteins (9). Thus, it is important to adopt proper strategies to prevent severe apoptosis and associated damage to support the myocardium, even in adolescence and middle age.
Recently, the molecular effects of different exercise modalities, especially high-intensity interval training (HIIT) programs, on the process of apoptosis has received the scholar’s attention. This type of exercise consists of short high-intensity efforts (usually > 90% VO2max) interspersed with low-intensity or resting intervals (10). Although studies showed that interval exercises could reduce cardiovascular risk factors (11, 12), there is a discrepancy regarding its effects on myocardial apoptosis. Some researchers believe that exposure to higher stress during exercise may accelerate the process of apoptosis and its consequences by increasing pre-apoptotic mediators or decreasing anti-apoptotic factors (13, 14). In contrast, the results of some studies indicate the protective role of exercise training against myocardial apoptosis (15-17). Lu et al. (18) recently indicated that HIIT was more effective than a moderate-intensity exercise to improve cardiac function, oxidative stress, and apoptotic markers in rats with myocardial infarction. Yet, the possible molecular alterations and protein responses to this exercise program have been less investigated, and the potential effect of HIIT on the process of apoptosis in myocardial tissue remains to be elucidated.
2. Objectives
The objective of the study was to determine the effect of 12-week HIIT on some markers of apoptosis in myocardial tissue of male rats.
3. Methods
3.1. Animals
In an experimental study, 30 male Wistar rats (age range of 8 - 10 weeks) were housed in a well-controlled temperature of 20°C - 24°C and humidity of 50 ± 10% under a 12/12 h light/dark cycle. The animals were allowed to feed ad libitum during the study. The study complied with the principals of the declaration of Helsinki, and the ethical approval was issued by the Research Ethics Committee of the Tabriz University of Medical Sciences.
3.2. Exercise Protocols
During the first week, animals were familiarized with the exercise protocol on a motorized treadmill (0%, 10 - 15 m.min-1, 5 - 10 min.d-1) for one week. Then, an incremental exhaustive exercise test was implemented to assess the maximal speed, commenced with 10 m.min-1, with an increase of 3 m.min-1 every 2 min until failure. Failure was defined when the rats could not continue running despite electric shocks and when they were unable to upright themselves when placed on the back (19). After the exercise test, animals were matched based on the weight and were randomly assigned into three groups: (1) Sham (n = 10), (2) control (n = 10), and (3) HIIT (n = 10). The sham group was anesthetized with ketamine and xylazine and sacrificed at the beginning of the study period.
Table 1 presents the exercise protocol from baseline to week 12, consisting of 2 - 8 repetitions of 4-min high-intensity intervals (85% - 90% peak speed), which interspersed with low-intensity intervals (45% - 50% peak speed) performed five times per week over 12 weeks (19). The exercise intensity increased by 5% each week.
| Week of Training | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High-intensity intervals | ||||||||||||
| Speed, m/min | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 |
| Time, min | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Low-intensity intervals | ||||||||||||
| Speed, m/min | 14 | 15 | 16 | 16 | 17 | 17 | 18 | 18 | 19 | 19 | 20 | 21 |
| Time, min | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Number of repetitions | 2 | 3 | 5 | 6 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Total workout time, min/day | 12 | 24 | 40 | 48 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 64 |
| Treadmill grade, % | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
The High-Intensity Interval Training Protocol
3.3. Tissue Removal
Anesthetization was applied by the intraperitoneal (IP) injection of ketamine and xylazine (90 mg.kg-1 and 10 mg.kg-1, respectively). They were then sacrificed 48 h following the last exercise session. The cardiac tissue was extracted and rinsed with the ice-cold physiological saline solution, and then the left ventricle was isolated. The tissues were then frozen in liquid nitrogen and maintained at -80°C for later analyses.
3.4. RT qPCR
The messenger (m) RNA levels of Bax, Bcl-2, and caspase-3 were evaluated by RT-qPCR. In this method, 50 mg of the left ventricle tissue was homogenized in lysis buffer, and the total RNA was extracted and reverse-transcribed into cDNA using the revert AIDTM Firs Standard cDNA synthesis kit (Fermentas, USA) with an Oligo dT primer. The real-time PCR was performed by SYBR® Premix Ex TaqTM II (Takara, Japan) following the instructions issued by the manufacturer. The mixture included 10 µL of SYBR green mix, 1.2 µL of cDNA (equivalent to 1 ng of the total RNA with an initial concentration of 100 ng.µL-1), 0.4 µL of each of forward and reverse primers at 10 pmol.µL-1 concentration, and Millipore® water to achieve a final volume of 20 µL. Table 2 shows the primer sequences. For each thermal cycling set, the threshold cycle (CT) was manually determined. The efficiency of PCR for each set of primers was determined by 10-fold serial dilutions of cDNA and CT plots against logarithmic cDNA dilutions based on the efficiency formula of E = 10(-1/slope). The melting curve analysis was carried out in one cycle of 95°C for 5 s, 67°C for 25 s, and 99°C for 0 s with a ramp rate of 0.1°C s-1 and 55°C for 30 seconds. The mRNA quantification was conducted as a value concerning the internal reference of β-actin. Gene expression was calculated compared to controls based on the 2-ΔΔCt method using REST© software.
| Gene | Primer Sequence | Product Length, bp |
|---|---|---|
| Bcl2 | F: 5’TATATGGCCCCAGCATGCGA3’ | 136 |
| R: 5’GGGCAGGTTTGTCGACCTCA3’ | ||
| Bax | F: 5’ATCCAAGACCAGGGTGGCTG3’ | 150 |
| R: 5’CACAGTCCAAGGCAGTGGGA3’ | ||
| Caspase-3 | F: 5’GGAGCTTGGAACGGTACGCT3’ | 118 |
| R: 5’AGTCCACTGACTTGCTCCCA3’ | ||
| β-actin | F: 5’CTCTGTGTGGATCGGTGGCT3’ | 138 |
| R: 5’GCAGCTCAGTAACAGTCCGC3’ |
Primers Used in Real-Time Polymerase Chain Reaction of Gene Expression Analysis
3.5. Statistics
The means and standard deviation (SD) of data are presented in tables. The normal distribution of the data was confirmed by the Shapiro-Wilk statistical test. Thus, a parametric test (independent t-test) was applied to assess between-group differences. Statistical significance was determined if P < 0.05. The analyses were performed using SPSS version 19 for Windows.
4. Results
The mean ± SD of the variables is shown in Table 3. The results showed that following the 12-week exercise, the body weight was significantly lower (P = 0.029) and the heart weight (P = 0.34; ~8%) and heart/body weight ratio (P = 0.001) were significantly higher in the intervention group than in the control group.
| Sham (N = 10) | CON (N = 10) | HIIT (N = 10) | |
|---|---|---|---|
| Baseline body weight, g | 318.34 ± 19.38 | 322.12 ± 21.57 | 320.09 ± 19.57 |
| Final body weight, g | 381.13 ± 26.8 | 385.25 ± 29.6 | 340.33 ± 28.11b |
| Heart, g | 1.04 ± 0.11 | 1.06 ± 0.13 | 1.15 ± 0.17 |
| Heart/body weight, g.kg-1 | 2.73 ± 0.18 | 2.77 ± 0.22 | 3.36 ± 0.31b |
| Food intake, g.d-1 | 23 ± 2 | 23 ± 2 | 24 ± 2 |
Characteristics of Ratsa
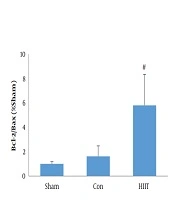
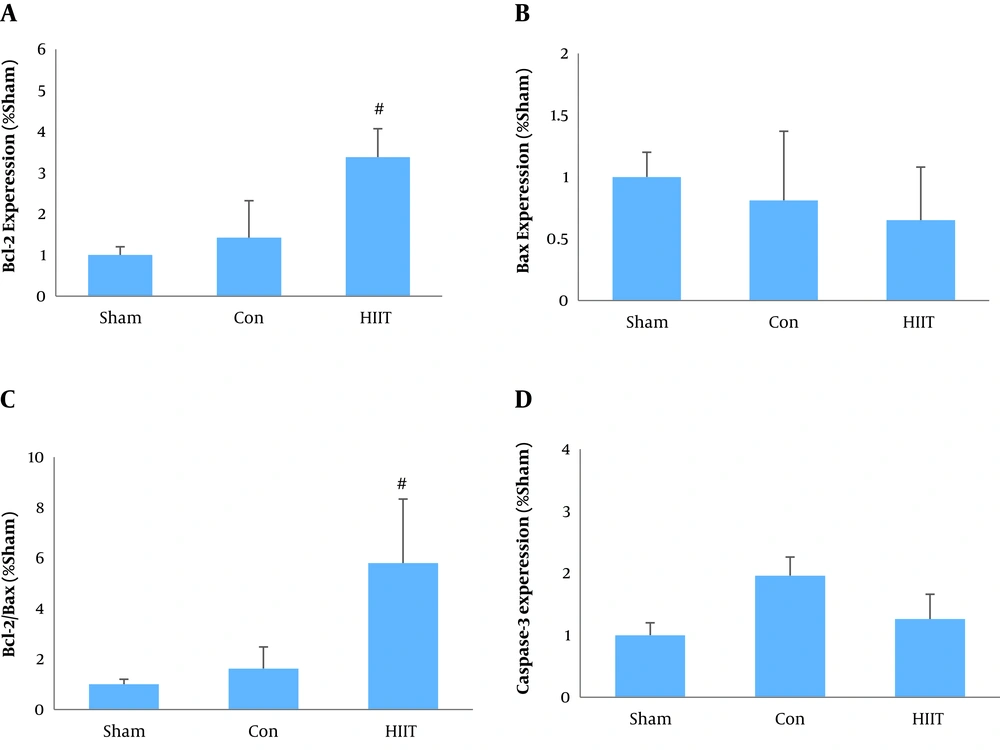
Regarding the gene expression of Bcl-2 and Bax, the data showed that the expression of Bcl-2 and the ratio of Bcl-2/Bax were significantly higher in the training group (P = 0.001 and P = 0.002, respectively). Regarding Bax and caspase-3 gene expression, although the mean levels were lower in the exercise group than in the control group, the differences were statistically insignificant (P = 0.57 and P = 0.27, respectively) (Figure 1).
5. Discussion
Excessive apoptosis in the myocardium contributes to the pathogenesis of heart disease. We investigated the effect of 12-week HIIT on some markers of apoptosis, including Bcl-2, Bax, and caspase-3 in the myocardial tissue of male rats. A significant reduction (12%) was observed in body weight following the exercise intervention when compared to the control condition. Furthermore, the heart weight and the heart/body weight ratio were about 8% and 21% higher in the HIIT group than in the control group, respectively, although the difference between the groups regarding the heart weight did not reach the significance level. As expected, the experimental group experienced a lower-extent weight gain than the control group. This is explained by the elevated calorie expenditure by exercising. It has been shown that HIIT increases the total body fat oxidation and the activity of linked enzymes (12) and suppresses appetite that consequently helps in weight control (20).
The main finding of the present study was that the Bcl-2 gene expression and the Bcl-2/Bax ratio were significantly higher in the HIIT group by about 138% and 258%, respectively. Even though the mean levels of Bax and caspase-3 gene expression were 20% and 36% higher in the experimental group than in the control group, the differences did not reach the statistical significance level. Very few studies have investigated the effect of HIIT on myocardial apoptosis, and the results are often inconsistent. In line with our findings, Lu et al. (18) reported that eight weeks of HIIT or moderate-intensity continuous exercise training elevated the expression of Bcl-2 and decreased the gene expression of Bax and caspase-3 in rats with myocardial infarction. They suggested that although there was no significant difference between HIIT and moderate-intensity continuous training in apoptosis, the HIIT program was more potent to reduce oxidative stress and improve the glycolipid metabolism. Gahramani et al. (21) also indicated that six weeks of HIIT significantly reduced the caspase-9 gene expression in rats with myocardial infarction. Although several mechanisms, including alterations in apoptotic gene expression, decreases in the release of mitochondrial apoptotic factors, changes in ROS generation and antioxidant status have been proposed for the protective effect of exercise training against apoptosis (22), they need to be further elucidated.
Mitochondrial adaptations appear to play a significant role in regulating apoptosis in tissues such as the myocardium (23). In this regard, the Bcl-2 family, including Bax and Bcl-2 proteins, are involved in the formation of apoptotic channels, regulation of mitochondrial permeability, and mitochondrial apoptotic signals. It has been established that the Bax protein enhances the activity of apoptotic signaling pathways (5, 8). Research indicates that Bax translocation to the mitochondria can induce apoptosis. This occurs via conformational changes that permit the Bax-Bax oligomerization and its insertion into the outer mitochondrial membrane, following which the release of the apoptogenic factors from the mitochondrial intermembrane space increases. In contrast, by preventing Bax-Bax oligomerization, Bcl-2 hinders the pro-apoptotic action of Bax. Moreover, the Bcl-2 protein enters the mitochondrial outer membrane and maintains the membrane integrity by discharging H+ ions through ion channels and inhibits the caspase activation by binding to Apaf-1 (4, 8, 23, 24). Thus, the Bax to Bcl-2 ratio is an indicator of the potential of mitochondrial apoptosis. Vainshtein et al. (25) suggested that mitochondrial apoptosis is often associated with an increase in reactive oxygen species, and exercise training can attenuate apoptosis by reducing the ROS production and promoting the antioxidant defense. Translocation and incorporation of the Bax protein into the mitochondrial outer membrane may increase in response to oxidative stress and elevated p53 protein, which may partly be due to the activation of cytosolic c-Jun-N-terminal kinase (JNK) such that JNK is phosphorylated by cellular stress stimuli and inhibits the Bcl-2 protein. This enables the Bax protein to shift to mitochondria. The JNK protein in mitochondria contributes to the opening of mitochondrial permeability transition pore (mtPTP) and releases pro-apoptotic factors such as cytochrome C and AIF into the cytosol. They cause DNA fragmentation directly or through the caspase cascade upon their release to the cytosol. This is associated with decreased expression and release of cytochrome C in the cardiac muscle of trained rats, representing the inhibition of apoptotic signaling (25). In addition, Lai et al. (26) reported that exercise training inhibits myocardial apoptosis and increases life span via activation of specific signaling pathways. Exercise activates downstream proteins (e.g., PGC-1α) and improves mitochondrial function by enhancing SIR1 proteins and activating their signaling pathway, leading to the inhibition of myocardial apoptosis. They noted that exercise training inhibited apoptosis via the SIRT/PGC-1α signaling pathway rather than thee IGF1R/PI3K/Akt pathway (26). It was a limitation to the present study that we did not assess the changes in SIRT proteins and their relationships with apoptotic proteins. It would provide a deeper insight into the involved apoptotic factors, and remains to be noticed in the future.
In contrast, our findings are inconsistent with some previous studies. A major reason for the discrepancy could be the exercise protocol as previous studies mostly investigated the acute effects of HIIT or continuous exercise. In this regard, Kruger et al. (14) showed that short-term HIIT exercise exacerbated apoptosis in the immune system. Unlike acute exercise, exercise training with long duration seems to bring about different physiological and metabolic adaptations, which can eventually affect the process of apoptosis. Contrary to our results, Yazdanparast Chaharmahali et al. (27) reported that eight weeks of HIIT training significantly increased the Bax gene expression and Bax/Bcl2 ratio in aged rat myocardium. Animals’ age seems to be the main reason behind this contradiction. Myocardial apoptosis is age-dependent, and cardiac cell death increases with age. Some studies showed that cardiac apoptosis was about 200% higher in 24-month-old mice than in 16-month-old mice, while no difference was observed in necrosis (28). Therefore, it is likely that intense training programs such as HIIT in the short term can increase mechanical and metabolic stress to the myocardial muscle and enhance the expression of pro-apoptotic proteins in the elderly. Yazdanparast Chaharmahali et al. (27) suggested that, unlike HIIT, moderate-intensity exercise could increase the Bcl-2 gene expression in the myocardium of aged rats. Liu et al. (13) also examined the effect of nine weeks of continuous and exhaustive endurance training on Bcl-2 and Bax gene expression in male rats and reported a significant increase in the Bax gene expression and an insignificant decrease in Bcl-2 (13). The discrepancy in our results and those reported by Liu et al. (13) seems to lie in the difference in the type and intensity of the training program. Liu et al. (13) applied a high-intensity continuous exercise intervention until failure, which could increase oxidative stress, as indicated by the elevation of MDA and XOD and reduction of SOD, resulting in augmented myocardial apoptosis.
5.1. Conclusions
Overall, we conclude that besides functional effects such as weight loss and increased heart/body weight ratio, HIIT significantly increases anti-apoptotic gene expression and anti-apoptotic/pro-apoptotic gene expression ratio in the myocardium. However, many other aspects of the HIIT effects on myocardial apoptosis, including oxidative/anti-oxidative status, other signaling pathways of apoptosis, and other proteins involved in mitochondrial apoptosis remain to be explored in future studies.