1. Background
Thyroid hormones have an important role in normal bone development and metabolism (1). Today, it has been cleared that bone marrow stromal cells (BMSCs) have receptors for the thyroid hormones, and so, BMSCs are T3-responsive cells (2). In addition, previous studies showed that the lack or decrease of thyroid hormones can impair in vitro osteogenic differentiation of the mesenchymal stem cells (MSCs) (1, 3).
Adipose-derived mesenchymal stem cells (ADMSCs) are known as available and abundant cell sources of the adult stem cells (4). Unlike BMSCs, they have many advantages, such as ease of harvest, abundance, higher cellular yield, low immunogenicity, and less amount of the required factors for their growth (4-7).
It is well established that MSCs secrete a wide range of growth factors and cytokines that exert paracrine effects on the other cell types and tissues (8). These factors can accumulate in the medium where MSCs. are cultured; therefore, that is called conditioned medium (CM) (9). Because of the limited survival and differentiation of transplanted MSCs, it has been suggested that MSCs paracrine signaling is the primary mechanism of their beneficial effects on tissue regeneration (10). Many cell types respond to MSCs paracrine signaling, which can regulate a number of cellular responses, such as cell survival, proliferation, migration and gene expression (11). Chen et al. have reported MSCs-CM can improve in vitro proliferation and migration of the alveolar epithelial cells via the JNK-P38 signaling pathway (12). Osugi et al. (13) demonstrated that the cell proliferation and expression level of the osteocalcin and Runt-related transcription factor2 (RUNX2) genes were significantly higher in the cultured MSCs with MSC-CM than the control group.
Many cytokines, such as insulin growth factor-I (IGF-I), Vascular endothelial growth factor (VEGF), transforming growth factor beta-1 (TGF-β1), and bone morphogenetic protein-1 (BMP-1) have been detected in the MSCs-CM, which can cooperate in the bone regeneration. Therefore, MSCs secretome has a high potential for osteogenesis (13-16). These factors regulate osteogenesis, angiogenesis, cell migration, osteoblast proliferation, and differentiation (10).
Cell therapy is not successful in the bone defects repairment of the hypothyroidism patients, due to the reduced osteogenic potential of autogenic MSCs. Thus, a different method must be considered. Levothyroxine is used to normalize the hormone levels; it can cause a few complexities, such as increased fracture risk during the first year of the levothyroxine therapy (17).
2. Objectives
Therefore, we hypothesized that the application of ADMSCs-CM may improve the cell proliferation and osteogenic differentiation of the hypothyroid BMSCs. Previous studies evaluated the effect of the MSCs-CM on the normal MSCs. To the best of our knowledge, no studies have been conducted using ADMSCs-CM to improve the proliferation and osteogenic differentiation of the hypothyroid MSCs.
3. Methods
3.1. Animals and Hypothyroidism Induction
The experiments were conducted using male Wistar rats. The animals were kept at a constant temperature of 21°C with a 12-h light/12-h darkness cycle. They received drinking water and a standard rat food pellet chow ad libitum. Hypothyroidism in rats was induced by administration of 4 mg Methimazole (Tehran, Iran hormone), dissolved in100 cc distilled water during 60 days according to the instructions of our previous study (18). The serum level of T4 and TSH hormones were measured by radioimmunoassay kit. The rats in the control group received drinking water without Methimazole.
3.2. Adipose Stem Cell Extraction and Conditioned Medium Preparation
About six to eight weeks, old male Wistar rats were sacrificed using chloroform inhalation. Adipose tissue from rat testicular fat pads was digested with collagenase type I (Sigma-Aldrich). After that, the centrifuge was conducted at 1500 rpm for 5 min, the supernatant was removed and the cell pellet cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco (supplemented with 10% fetal bovine serum (Gibco) and 100 mg/mL streptomycin and 100 U/mL penicillin (Gibco). The cells were incubated at 37°C in a humidified atmosphere of 95°C and 5% CO2. At passage 4, when confluency reached about 80% - 90%, the complete medium was replaced by serum-free DMEM and incubated for 48 h. Then, the conditioned media was collected and concentrated 20 fold using Amicon Ultra Centrifugal Filter Device (Millipore) with a 3 kD molecular weight cutoff.
3.3. Isolation of BMSCs and Experimental Group Design
The normal and hypothyroid animals were sacrificed by chloroform inhalation. The bone marrow was flushed out from tibia and femur with DMEM and then seeded into a 25 cm2 culture flask. The BMSCs at the third to fourth passages were assigned into experimental groups including group I: healthy control BMSCs in basal medium (DMEM + 10% FBS), group II: hypothyroid BMSCs in basal medium and group III, hypothyroid BMSCs in basal medium supplemented with 10% ADMSC conditioned media (ADMSC-CM).
3.4. Immunophenotyping of ADMSCs and BMSCs by Flow Cytometry
After three passages, the expression of surface markers of BMSCs and ADMSCs was evaluated using monoclonal antibodies fluorescein isothiocyanate (FITC)-conjugated or phycoerythrin (PE)-conjugated against CD45, CD34, CD105, CD90, and CD73 (eBioscience, San Diego, CA, USA). Cells were suspended with trypsin/EDTA, incubated in the darkness with the specific or isotype control antibodies and then analyzed with a flow cytometer (Attune ABI, USA) and FLOWJO software.
3.5. MTT Assay
The effect of ADMSC-CM on the hypothyroid BMSC proliferation was evaluated with MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazoliumbromide] assay (Sigma-Aldrich). BMSCs of all three experimental groups were seeded with a density of 5 × 103 cell/well in 24- well tissue culture plates (TCPS). After 1, 3, and 5 days from cell seeding, 200 µL MTT solution (5 mg/mL in DMEM) was added to each well to allow formazan crystals formation by the living cells mitochondria. The plates were incubated at 37°C for 3 h. Then, for the dissolution of formazan crystals, the medium was replaced with 200 µL of DMSO (Gibco), and the absorbance was measured at 570 nm.
3.6. Osteogenic Differentiation and Evaluations
For osteogenic evaluation assays, all the three groups above were cultured under osteogenic differentiation media (DMEM supplemented with 10 % FBS, 10 -7 M dexamethasone, 10 mM b-glycerophosphate, and 50µg/ml ascorbic acid 2-phosphate) for 21 days. Moreover, the hypothyroid-CM group received 10% ADMSC-CM in osteogenic media. Medium was changed every 3 days.
3.7. Alizarin Red S Histochemical Staining
On day 21, Alizarin red staining was used to evaluate the mineralized matrix in the groups. The medium was removed and the cells were washed with cold PBS three times and fixed in 4 % paraformaldehyde for 20 min at 4°C. Then, the cells were washed with PBS twice and were stained with 2% Alizarin red S at pH = 7.2 (Sigma). After 5 - 10 min, at 37°C, the cells were washed again with PBS three times and examined by light microscopy.
3.8. Alkaline Phosphatase Activity
For ALP investigation, the total protein of cells were extracted at 7, 14, and 21 days of osteogenic differentiation using 200 µL radio immune precipitation assay (RIPA) lysis buffer. Then, the protein solution was centrifuged at 15000g at 4°C for 15 minutes, and the supernatant was collected. ALP activity was measured colorimetrically using an ALP Kit (Parsazmun, Tehran, Iran) with p-nitrophenyl phosphate (pNPP) as a phosphatase substrate. The activity of the enzyme (OD) was normalized against total protein (mg), measured by BCA protein assay kit (Thermo Fisher Scientific, USA).
3.9. Calcium Content Assay
The calcium deposition on TCPS was extracted with 0.6 N HCl (Merck) followed by shaking for 1 h at 4°C. Then, the reagent (Calcium Content AssayKit, Pars Azmoon, Iran) was added to calcium solution, and the optical density was measured at 570 nm. Calcium content values of samples were calculated based on the standard curve of OD versus serial dilution of calcium concentrations.
3.10. Real-Time RT-PCR
Quantitative gene expression of Collagen type I alpha 1 (Col-1a1), Runt-related transcription factor 2 (Runx2), and osteocalcin (Ocn) in the osteogenic differentiating BMSCs of all three groups were analyzed at 7th and 14th day via real-time RT-PCR. Total RNA was extracted, and random hexamer-primed cDNA synthesis was conducted using Revert Aid first-strand cDNA synthesis kit (Fermentas, Burlington, Canada). The PCR conditions were as follows: 95°C for 2 min, then 35 cycles at 95°C for 5 s, 60°C for 30 s. The sequence of the specific primers used for PCR amplifications are illustrated in Table 1. Real-time RT-PCR was performed using Maxim SYBR Green/ROX qPCR Master Mix (Fermentas) followed by a melting curve analysis to confirm PCR specificity. The relative expression was quantified using the ΔΔCt method. Target genes were normalized to HPRT expression. Data were analyzed via REST 2009 software.
| Gene | Primer Sequence (F, R) | Product Length, bp |
|---|---|---|
| HPRT1 | CCAGCGTCGTGATTAGTG | 135 |
| CGAGCAAGTCTTTCAGTCC | ||
| COL1A1 | AGAAGAATATGTATCACCAGACG | 190 |
| AGCAAAGTTTCCTCCAAGAC | ||
| RUNX2 | GAAATGCCTCTGCTGTTATG | 191 |
| TCTGTCTGTGCCTTCTTGG | ||
| BGLAP(OCN) | TCAACAATGGACTTGGAGCC | 160 |
| CAACACATGCCCTAA ACGG |
3.11. Statistical Analysis
All the experiments were conducted at least three times. A one-way analysis of variance (ANOVA) was used to compare the results. P value < 0.05 was considered as a significant level, and data were expressed as mean ± SD. The statistical analysis was performed by SPSS software, version16 (SPSS, Chicago, IL, USA).
4. Results
4.1. Hypothyroidism Induction
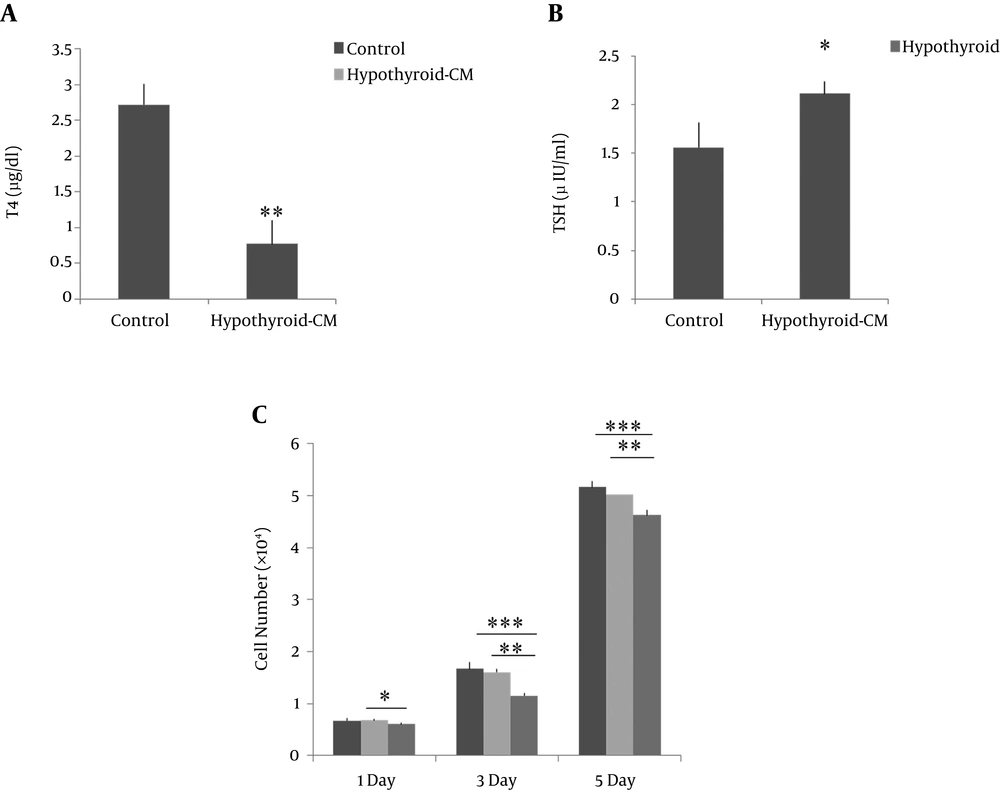
The induction of hypothyroidism was confirmed through analyzing the serum concentrations of T4 and TSH (Figure 1A and B). T4 serum level was significantly decreased in the hypothyroid rats in comparison with control; TSH serum level was significantly increased in the hypothyroid rats in comparison with control.
4.2. Characterization of Isolated BMSC and ADMSC
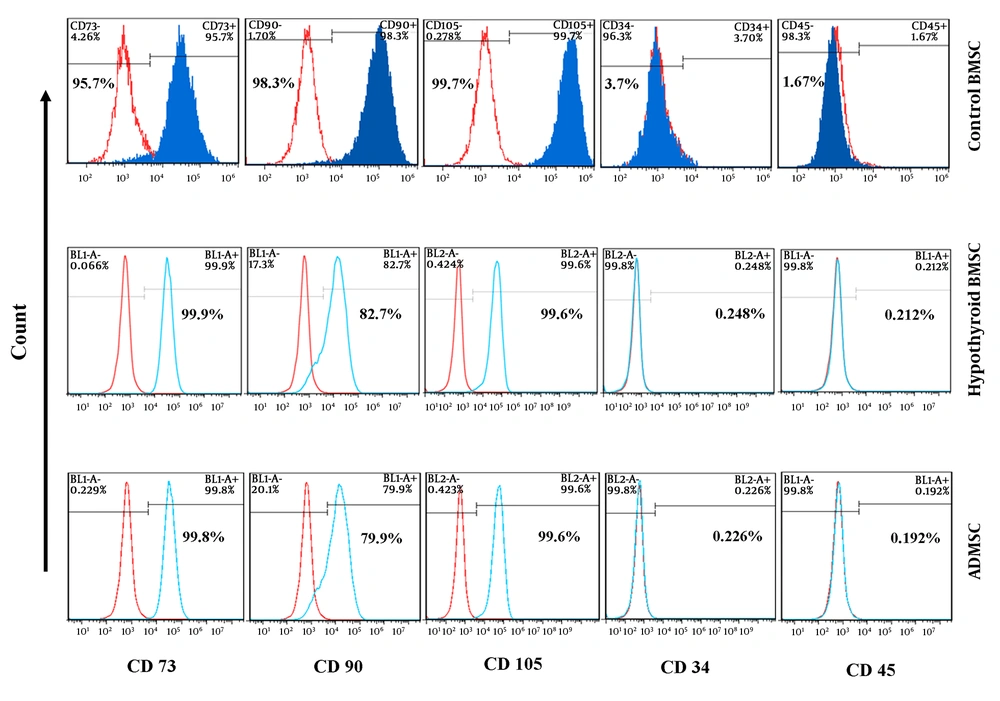
Isolated stem cells from the adipose tissue and bone marrow were characterized based on their morphology and expression of the surface markers in them. Both types of these stem cells were showed fibroblast-like and spindle-shaped morphology when cultured in a low cell density. Flow cytometric analysis showed the absence of the specific hematopoietic cells markers, CD34 and CD45, while all the stem cells were positive at the high percentage for specific MSCs markers such as CD73 and CD90 and 105 (Figure 2).
4.3. Effect of ADMSC Conditioned Media on hypothyroid BMSC Proliferation
To evaluate the effect of ADMSCs condition media on the hypothyroid BMSCs proliferation, the cell numbers were estimated after 1, 3, and 5 days after cell seeding by MTT assay. According to the results, ADMSCs-CM significantly enhanced the cell proliferation of hypothyroid MSCs at the three evaluated days compared to non-treated cells. There was no significant difference between hypothyroid-CM and control groups in each day (Figure 1C).
4.4. Osteogenic Differentiation: Alizarin Red Staining, ALP Activity, and Calcium Content
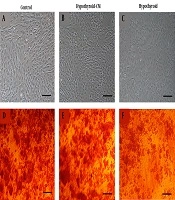
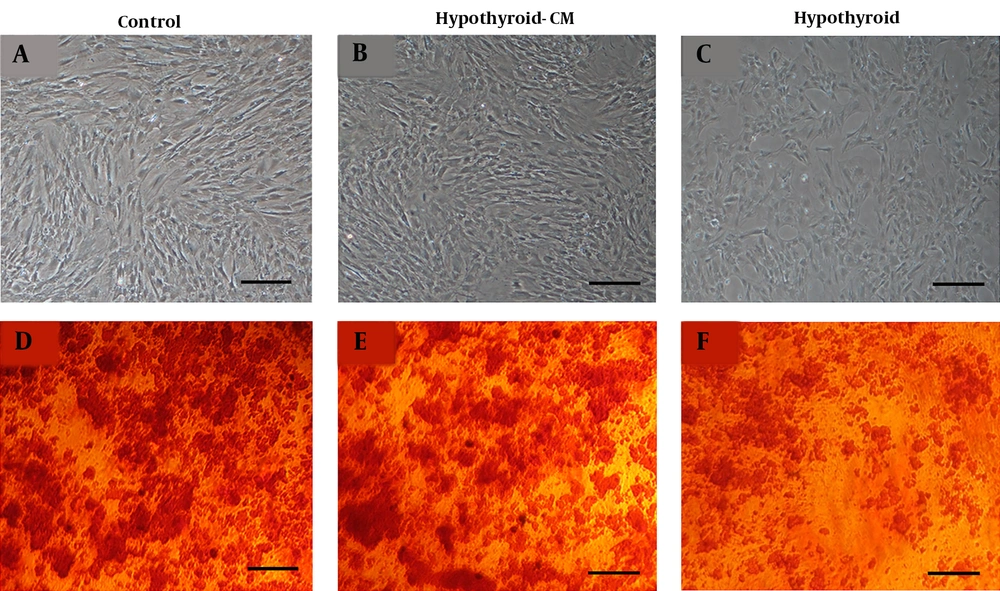
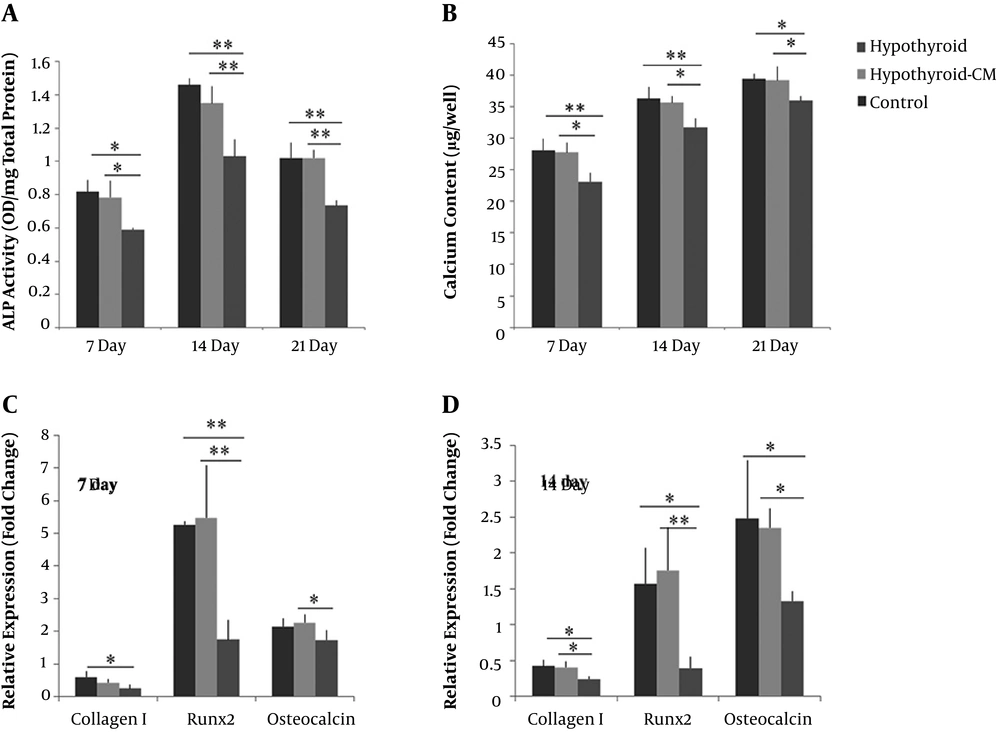
The mineralization of three BMSCs groups was first confirmed by alizarin red staining on day 21. A lower amount of mineralization was observed in the hypothyroid compared to the control and hypothyroid-CM groups (Figure 3). ALP activity of BMSCs was measured at three-time points during osteogenic differentiation. The highest amount of ALP activity was observed in all three groups on day 14. Remarkably, the ALP level in the hypothyroid group was lower than the control and hypothyroid-CM groups.
On the other hand, the hypothyroid-CM group showed no significant difference with the control group. Thus, the condition media increased the ALP activity of hypothyroid BMSC during osteogenesis (Figure 4A). Calcium deposition of BMSCs increased continually during the osteogenesis induction. In all time points, the hypothyroid group showed a significantly lower amount of mineralization compared to the control and hypothyroid-CM groups. The hypothyroid-CM was not different from the control group at 7, 14, and 21 days (Figure 4B).
4.5. Osteogenic Gene Expression Analysis
The effect of the ADMSCs-CM on the relative expression of the osteogenic markers of hypothyroid BMSCs was measured during osteogenic differentiation (Figure 4C). The osteocalcin expression tended slightly to rise during induction time in the control and hypothyroid-CM. The expression of RUNX2 gene peaked on day 7, followed by a decrease on day 14. Hypothyroid BMSCs showed a significantly lower expression of osteocalcin and RUNX2 on day 7 compared to hypothyroid-CM. Relative expression of collagen I and RUNX2 was down-regulated in the hypothyroid group on day 7 compared to control. The expression levels of these three genes were significantly down-regulated in the hypothyroid BMSCs on day 14 compared to other groups. At two time points, hypothyroid-CM showed no significant difference with control BMSCs.
5. Discussion
We found that the proliferation level of hypothyroid BMSCs was lower than normal BMSCs; our data indicated that ADMSCs-CM improved the hypothyroid BMSCs proliferation. As expected, the proliferation of BMSCs was significantly increased in the presence of ADMSCs-CM, because of some growth factors such as FGF-2 and platelet-derived growth factor (PDGF) known to induce cell proliferation in MSC secretion (9, 19). In this regard, Park et al. (20) have demonstrated that ADMSCs-CM increased the proliferation of human follicle dermal papilla cells (HFDPCs) and human epithelial keratinocytes. ALP is a key enzyme during osteogenesis. Lesse osteogenic potential of hypothyroid BMSCs can be concluded from the lower ALP activity compared to the normal and hypothyroid-CM BMSCs. Furthermore, the amounts of calcium content confirmed the patterns of ALP activity in three experimental groups. In the following, we attempted to evaluate the effects of ADMSCs-CM on the osteogenic differentiation of hypothyroid BMSCs by analyzing three important bone-related gene markers. According to the results, the expression levels of collagen I, RUNX2, and osteocalcin were decreased in hypothyroid BMSCs compared to control and hypothyroid-CM. A study by Boeloni et al. (1) showed that collagen I down-regulated in the first week of osteogenic induction in hypothyroid BMSCs. Compared to the other two genes, collagen I expression remained at a lower level during the induction period. Some studies have shown that glucocorticoids inhibit transcription of collagen mRNA and so, downregulate its gene expression (21, 22). Our results showed that the hypothyroidism reduced the osteogenic differentiation of the bone marrow MSCs. Consistent with our results, Simsek et al. (3) have reported that the stem cells derived from thyroidectomized rats had a lower alkaline phosphates activity and mineralization. Also, lack of osteocalcin and osteopontin expression was shown in them compared to the normal stem cells. Osteogenic potential could be reduced due to the low proliferation rate of hypothyroid BMSCs, because the confluency of stem cells is very important in osteogenic differentiation (23).
Although the use of the growth factors, such as BMPs, TGF-β, and FGFs play a vital role in the osteogenesis (13, 24), these factors are so expensive, which can exacerbate the inflammatory responses. In addition, the use of a single growth factor limits the ability of the osteogenesis process (13). Therefore, this study employed the ADMSCs-CM as a combination of different growth factors. The results indicated that ADMSCs-CM increased the ALP activity and mineralization of the hypothyroid BMSCs. Some growth factors such as IGF-1, IL-6, TGF-β, FGFs and PDGF involved in the osteogenesis process have been secreted by ADMSCs (10, 14, 18, 25). This secretion can promote the osteogenic capacity of BMSCs. Regarding the role of these factors, it can be said that IGF-1 is present in bone tissue causing osteoblasts proliferatation (26). TGF-β family induce the expression of collagen as one of the most important proteins in the bone extra cellular matrix (ECM) (27). TGF-β1 promotes osteogenesis by recruiting osteoprogenitor cells and inducing their proliferation and differentiation into osteocytes (28). It has been demonstrated that the use of FGF-2 increases the height of the alveolar bone and periodontal tissue regeneration (29).
Katagiri et al. (30) have reported that MSCs-CM enhanced cell migration, tube formation, expression of the osteogenic, and angiogenic genes of rat MSCs.
Osugi et al. (13) showed that the cell proliferation and expression of osteogenic markers were increased in MSCs cultured with MSCs-CM. The present study illustrated that hypothyroidism reduced cell proliferation and osteogenic differentiation potential of BMSCs. Also, ADMSC-CM could enhance the proliferation rate and osteogenic potential of hypothyroid BMSCs.
5.1. Conclusions
In conclusion, we suggest that the use of ADMSC-CM be a promising source for tissue engineering applications in bone regeneration of the hypothyroidism cases.
5.2. Limitations and New Suggestions
Due to financial limitations, the level of growth factors in the ADMSCs-CM was not measured. Also, the gene expression was not investigated on day 21 of the differentiation. It is suggested that these items be considered in the future studies. The use of hypothyroid stem cells is also recommended in hypothyroid bone defects.