1. Background
Oxidative stress due to the imbalance between increased production of free radicals and the ineffectiveness of antioxidant defenses play an important role in the development of many diseases, including cardiovascular disease (1). Meanwhile, hydrogen peroxide weakens the antioxidant mechanisms by causing poisoning and increasing oxidative pressure (2). This process, through the mediators of Ryadonin receptors, stimulates the release of Ca++ into the sarcoplasmic reticulum and causes muscle damage to the heart cells (3). In fact, after oxygenated water poisoning, the body’s peroxidation-antioxidant (PAB) balance is severely impaired. These chemical compounds induce oxidative stress by creating active oxygen species (ROS) or inhibiting antioxidant systems (4). Therefore, finding ways to restore antioxidant balance in order to prevent and treat diseases caused by oxidative stress seems necessary.
There is ample evidence that aerobic exercise reduces oxidative stress by adapting to antioxidant systems. These exercises increase the body’s ability to counteract anti-oxidation systems and protect the body against the destructive properties of indicators such as malondialdehyde (MDA) (5). This means that adaptation to exercise and the antioxidant effects of aerobic exercise have been confirmed in systematic animal and human review studies (6). On the other hand, performing a strenuous physical activity session increases the oxidative stress of the heart muscle by producing mitochondrial hydrogen peroxide (7). This has been well demonstrated in the study of short-term resistance training compared to long-term resistance training. Accordingly, researchers have linked the lack of alteration or reduction in the activity of antioxidant enzymes in aerobic exercise to intense, high-pressure exercise. Under such conditions, the increase in reactive oxygen species and the resulting oxidative stress limit the activity of antioxidant enzymes (8).
Athletes are now looking to use low-risk supplements or medications with minimal side effects to increase athletic performance, recovery, or rapid strengthening of the body’s systems. In this regard, it has been reported that the use of Tribulus terrestris (Tt) extract is effective in reducing oxidative stress (8). The shrub is a shrub of the genus Zygophyllaceae with about 30 genera and 235 species, most of which grow in the desert or temperate and tropical regions. The most important beneficial elements of this plant are saponins and flavonoids. These compounds have anti-inflammatory, antioxidant, anti-aging, blood-sugar-lowering, anti-clogging, anti-poisoning, androgen-enhancing properties, etc. (9-11).
In summary, several factors can affect the balance of oxidative defense. Meanwhile, by changing the type, intensity of exercise and nutrition of people, the amount of antioxidant and oxidative factors can be affected. Existing studies suggest that regular exercise can increase capillary density and protect against mitochondrial poisoning by affecting the expression of the gene for antioxidant enzymes (5, 6). Poisoning affects cell energy charge by affecting ATP production (2, 12). In fact, the involvement of defense mechanisms such as the cytochrome C opposition involved in ATP production in mitochondria is one of the reasons for this decrease in cellular energy charge (3, 13). On the other hand, the use of herbal supplements, especially Kharkhask extract, can be one of the ways to strengthen antioxidant defenses (8-11). Evidence suggests that antioxidant enzymes during exercise may not completely prevent oxidative damage, and in such cases, the role of dietary antioxidants is important (7). Therefore, trying to find the best and least risky way to reduce oxidative stress is essential. It is possible to prevent the side effects of hydrogen peroxide poisoning by supplementing Kharkhask extract with aerobic exercise. In this regard, limited studies have been conducted separately, and integrated information on the simultaneous use of aerobic exercise and Kharkhask alcoholic extract is not available.
2. Objectives
Therefore, this study aimed to investigate the effect of eight weeks of aerobic exercise and Kharkhask extract on the tissue concentration of cytochrome C, ATP, MDA, and PAB of cardiac tissue of male rats poisoned with hydrogen peroxide.
3. Methods
In an experimental study, 36 male Wistar rats of 10 to 12 weeks (220 g - 200 g) were selected from the statistical population of the study and randomly divided into seven groups: (1) control; (2) poisoning; (3) poisoning + exercise; (4) poisoning + spine 1 (5 mg); (5) poisoning + spike 2 (10 mg); (6) poisoning + exercise + spike 1; (7) poisoning + exercise + spike + 2 were divided. For a week before the animals were tested, on the shelves of rodents, clear polycarbonate floor mats with clean wooden chips and in an environment with a temperature of 22°C to 24°C, humidity of 50 to 55% and a dark cycle of 12:12 hours were maintained (14). During the research period, the animals had compressed and prepared food for mice and free water with special bottles. All rats after a period of adaptation to the new conditions for one week (5 sessions of walking on the treadmill at a speed of 15 meters per minute and a slope of zero degrees and for 20 to 40 minutes with how to work on the special treadmill Animals (Pishro Andisheh Sanat Model Company 2014) were introduced (15).
One week later, the target groups were fed 100 mg per kilogram of body weight of oxygenated water to induce oxidative stress (2, 16). The aerobic exercise program of the training groups, including eight weeks (5 days a week) running on the treadmill, was performed at a speed of 20 meters per minute for 60 minutes per session (between 8 and 10 AM). Each training session began with a warm-up program at the beginning of each session, including a 10-minute run at a speed of 15 meters per minute and a gradual increase in speed. The cooling operation is also performed at the end of each exercise, with a gradual decrease in speed at the end of each training session. During the experimental intervention, the control groups had no exercise activity and were kept in cages (17).
In this study, the percolation method was used to prepare Tribulus terrestris plant extract, and for this purpose, sufficient amounts of the plant were prepared. According to this method, after drying, the plant was powdered using an electric grinder, then a sufficient amount of the resulting powder was dissolved in 200 mg of 70% ethanol, and the resulting mixture was kept at room temperature for 24 hours. Then, with the help of a mixing machine, the mixture was uniformly formed and infiltrated by filter and concentrated by a rotary machine. Finally, using a desiccator, all moisture was removed from the mixture, and an extract with the desired doses was obtained (18).
Tribulus terrestris alcoholic extract with a dose of 5 and 10 mg per kg body weight was prescribed to target animals for two weeks as cow dung. At the end of eight weeks, all groups were anesthetized under completely similar conditions and under basic conditions (24 hours after the last session of training or prescribing) with ketamine 30 - 50 mg/kg, and their heart was taken from a 5 mL blood syringe. The blood was taken into special tubes with liquid nitrogen and then stored in the freezer for less than 30°C until the desired index was measured. After serum preparation, adequate measurement of tissue concentration of MDA, PAB, cytochrome c, and ATP indices was performed by special methods.
3.1. Statistical Analysis
The amount of MDA was measured using the R & D Systems method and with special measurement kits from the American company Minipolis. Accordingly, after collecting blood samples, 150 microliters of ethylene diamine tetraacetic acid (EDTA) was added to the blood samples, and then it was centrifuged at 2700 rpm for 7 min. Plasma and surface layers were then separated from erythrocytes and used to measure MDA (19).
To evaluate the peroxidant-antioxidant balance, the tetramethylbenzidine (TMB) cation was used as an oxidation-reduction index due to its electrochemical and optical properties. In an enzymatic reaction, the dye-producing TMB was oxidized to the colored cation by the peroxidants, and in a chemical reaction, the TMB cation was converted to a colorless compound by the antioxidants.
In summary, a standard curve was plotted using ratios of 0 to 100% 250 µM hydrogen peroxide with three mM uric acid. Based on the concentration of hydrogen peroxide in the reaction, the peroxide enzyme oxidizes the TMB substrate to blue. At the end of the reaction, hydrochloric acid causes a yellow color at a wavelength of 450 nanometers. The values of the peroxidant-antioxidant ratio in HK units were expressed based on the absorption of hydrogen peroxide percentage in the standard solution (20). Thus, the PAB values in the blood samples of the experimental groups were measured.
US-made cytochrome C oxidase (COX) ELISA kits are used to quantitatively evaluate the concentration of serum cytochrome C oxidase in plasma and tissue rats of laboratory rats. Accordingly, to measure this index, part of the heart, muscle tissue was isolated and weighed. The isolated tissue was then hemolyzed by a hemogenolysis device at 0°C. Then, to separate the mitochondria from the obtained solution, the centrifugation was performed in two turns by the refrigerator centrifugal device at -20°C. The mitochondrial proteins were then separated from the solution using the Lowry method, and finally, using the cytochrome C oxidase measuring kit and the spectrophotometer, the amount of tissue cytochrome C activity was measured (21).
KA1661 ATP evaluation kit was used to measure intracellular ATP levels. In this method, the activity of the luciferase enzyme of the desired solutions was determined by adding the kit substrate to the samples in the BIANlum model III luminometer of TIANLONG company. In this reaction, ATP, along with luciferin and oxygen, and in the presence of the enzyme luciferase, forms oxyluciferin complex and induces light emission. The light intensity produced is directly proportional to the amount of ATP and is expressed in units of µM/L (22).
After collecting the data and obtaining the mean and standard deviation of the data using descriptive statistics, the Shapiro-Wilk test was used to determine the normal distribution of data. Then, a two-way analysis of variance analysis and LSD follow-up tests were used to investigate the research hypotheses. All statistical operations were tested using SPSS software version 16 and at a significance level of 0.05.
4. Results
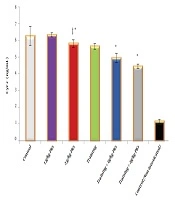
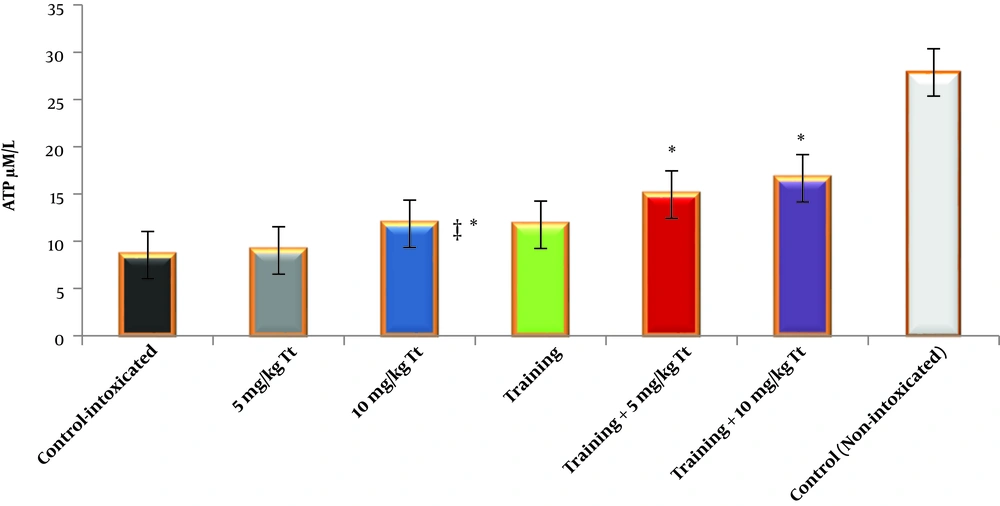
Training (F = 205.070, P = 0001.0, η = 0.872), Tribulus terrestris (F = 53.158, P = 0001.0, η = 0.780) and the interaction of training and Tribulus terrestris (F = 6.540, P = 0.004, η = 0.304) were effective on the ATP concentration of the heart tissue, with the highest effect on the combination of training and extract groups, especially H2O2+ training + Tt 2 group (Figure 1).
ATP concentration in the heart tissue in the studied groups. Data are reported based on mean and standard deviation. *‡, Indicates significant differences with the control groups. *, Indicates a significant interaction between training and Tribulus terrestris extract, especially at a dose of 10 mg/kg.
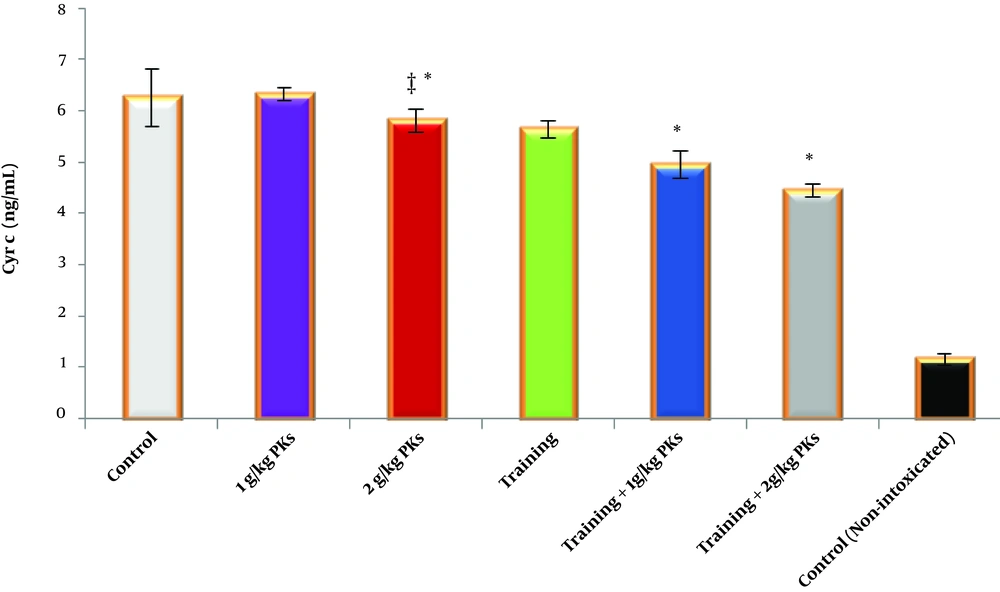
In addition, exercise (F = 50.401, P = 0001.0, η = 0.627) and thorns (F = 6.639, P = 0.004, η = 0.307) had a significant effect on tissue concentration of cytochrome C, but the interaction between exercise and thorns (F = 2.872, P = 0.072, η = 0.161) did not affect the concentration of cytochrome C in the cardiac tissue. Among these, the greatest effect has been observed in the groups of combination of exercise and extract, especially the group of poisoning + exercise + Tribulus terrestris 2 (Figure 2).
Cytochrome C concentration in the cardiac tissue in the studied groups. Data are reported based on mean and standard deviation. *‡, Indicates significant differences with the control groups. *, Indicates a significant interaction between training and Tribulus terrestris extract, especially at a dose of 10 mg/kg.
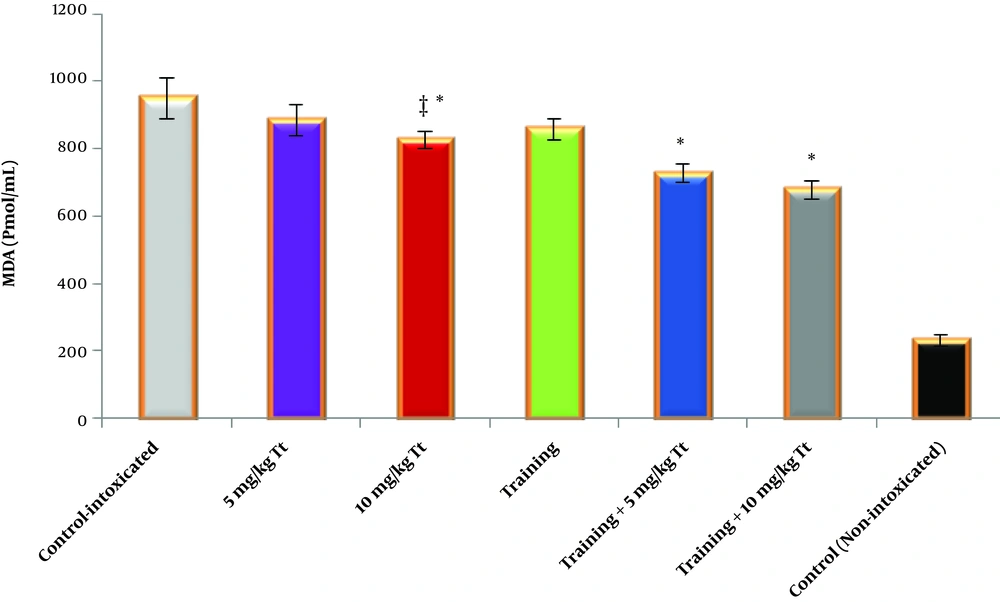
It was also found that adaptation to training had a significant effect on reduced concentration of MDA in the heart tissue (F = 70.558, P = 0001.0, η = 0.702). Also, receiving Tribulus terrestris extract had a significant effect on reduced concentration of MDA in the cardiac tissue (F = 28.327, P = 0001.0, η = 0.654). The interaction of training and Tribulus terrestris extract also showed a significant synergistic effect on reduced MDA concentration of the cardiac tissue (F = 2.064, P = 0.015, η = 0.121) (Figure 3).
Malondialdehyde (MDA) concentration in the heart tissue of the studied groups. Data are reported based on mean and standard deviation. *‡, Indicates significant differences with the control groups. *, Indicates a significant interaction between training and Tribulus terrestris extract, especially at a dose of 10 mg/kg.
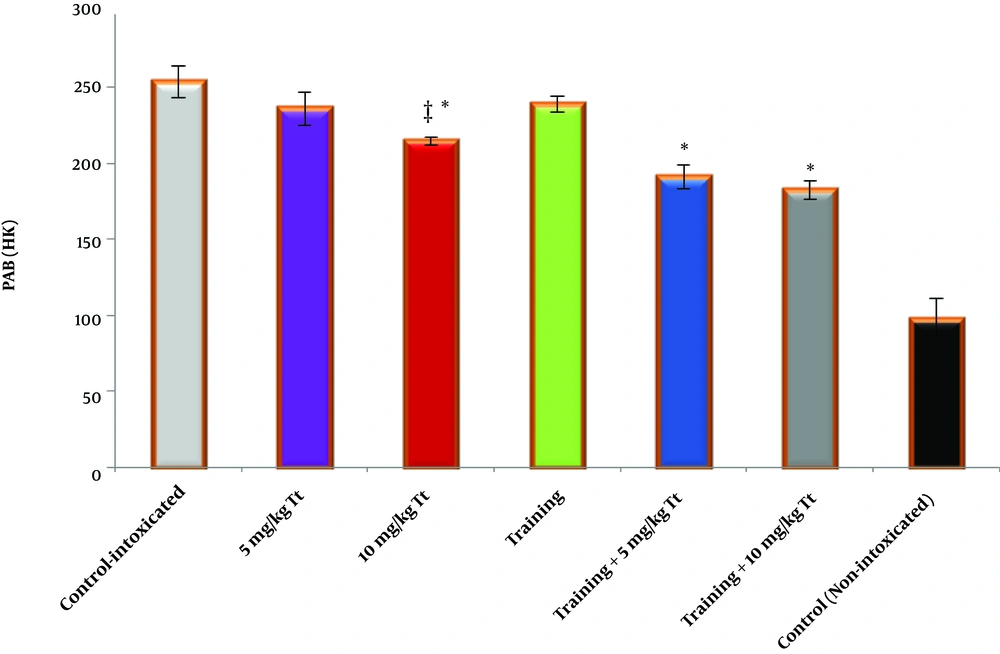
Based on the results obtained, training had a significant effect on reduced PAB (F = 30.271, P = 0001.0, η = 0.502). Also, receiving Tribulus terrestris extract had a significant effect on reduced PAB (F = 8.880, P = 0.001, η = 0.372). The interaction of training and Tribulus terrestris extract, especially in the dose -2 group (10 kg/mg) also had a significant effect on further reduced PAB (F = 5.276, P = 0.011, η = 0.260) (Figure 4).
Peroxidation-antioxidant (PAB) concentration in the cardiac tissue of the studied groups. Data are reported based on mean and standard deviation. *‡, Indicates significant differences with the control groups. *, Indicates a significant interaction between training and Tribulus terrestris extract, especially at a dose of 10 mg/kg.
5. Discussion
The aim of the present study was to investigate the simultaneous effect of aerobic exercise and Tribulus terrestris alcoholic extract on improving oxidative stress indicators in male Wistar rats poisoned with H2O2. For this purpose, Tribulus terrestris extract was used in dose 1 (5 mg/kg) and dose 2 (10 mg/kg) alone and in combination with aerobic exercise. In summary, in all cases, the tissue concentrations of cytochrome C, ATP, MDA, and PAB were significantly altered in the toxicity + exercise + exercise + thoracic dose of 10 mg compared to the other toxic groups. This change indicated a significant increase in ATP and a decrease in other variables. Also, practice and Tribulus terrestris separately had a significant effect on the dependent variables. However, the antioxidant defense performance of the poisoned groups was lower than that of the control group.
The results of a significant increase in APT index after combined use of eight weeks of endurance training and 10 mg supplements of Tribulus terrestris are consistent with the theoretical background of this issue. Accordingly, the most important physiological mechanisms for dealing with oxidative stress due to hydrogen peroxide poisoning are the effects on ROS, PI3K/Akt signal pathways, c (PKCs) protein kinase proteins, sodium-potassium pumps, and ATP-sensitive KPAT channels in the mitochondria (23, 24). Accordingly, the main route of induction of hydrogen peroxide poisoning is to reduce the activity of PKC signaling pathways (24), which will be addressed by the use of herbal supplements or long-term adaptation to aerobic physical activity or a combination of both. Myocyte stress can also be prevented by physical fitness, cardiovascular fitness, and special adaptations to regular physical activity, such as affecting PI3K (25).
In the study of the activity of the cytochrome C oxidase enzyme, this enzyme was significantly reduced in the experimental group of poisoning + exercise + thorn + ten mg compared to other groups. Because endurance activity increases mitochondrial density and the activity of oxidative enzymes in muscle tissue, this finding is somewhat interesting. However, it can be assumed that the enzymatic activity of mice in other groups has increased. In this regard, to explain these results, we can examine the structural changes of cytochrome C under the influence of exercise and supplementation. Studies show that many enzymes contain metal ions (iron in cytochromes) in a part of their structure. Therefore, the removal of metal ions is associated with structural changes and enzyme inactivation (26). Therefore, a decrease in the activity of cytochrome C oxidase in the group of poisoning + exercise + Tribulus terrestris 10 mg may be due to its poverty in this group. Apart from its important function as one of the components of hemoglobin, iron is a key factor in many enzymes, such as the III complex of the electron transport chain in mitochondria, which has undergone changes to adapt to the program of exercise programs in laboratory animals (27, 28). In this regard, Shetab-Boushehri et al. (26) examined the effect of endurance training and iron supplementation on some cellular respiration markers in rats and showed that the activity of cytochrome C oxidase enzyme increases significantly after 12 weeks of endurance training and iron supplementation. However, a definite statement in this regard requires further studies to compare the effects of taking Tribulus terrestris and iron supplements in combination with endurance activities.
Biochemically, increased tissue levels of malondialdehyde are one of the effects of exacerbation of oxidative stress due to increased free radicals of oxygen poisoning with hydrogen peroxide. The results of the present study showed that eight weeks of aerobic exercise had a significant effect on the tissue MDA factor of poisoned mice. These results are consistent with similar research in the human and animal spheres. Yeylaghi Ashrafi and Dabidi Roshan (29) in a review of domestic research showed that eight weeks of regular aerobic exercise is necessary to regulate the reduction of malondialdehyde. Similarly, in the study of Pourfazeli et al. (30), plasma levels of malondialdehyde index after 8 weeks of moderate-intensity aerobic exercise in the aerobic exercise group were significantly lower than the non-exercise group. Findings from Li et al. (31) also showed that aerobic exercise can increase adaptive responses to oxidative stress and promote MDA reduction in patients and rats. However, Homay et al. in a recent study titled the effect of aerobic exercise and L-carnitine consumption on some factors of oxidative stress in the tissue of all diabetic rats, showed that six weeks of aerobic exercise has no significant effect on malondialdehyde factor (32).
On the other hand, there are limited studies on the effects of thorn plants on the tissue and serum concentrations of malondialdehyde. Along with the results of the present study, Hammoda et al. (33) showed that Tribulus terrestris plant extract has hypoglycemic and hyperlipidemic properties and prescribing this plant reduces lipid peroxidation indicators and ultimately increases antioxidant levels. Also, the effect of resistance training and its combination with Tribulus terrestris water extract has been confirmed on the improvement of lipid profile (34). Sailaja et al. (35) reported the protective effect of Tribulus terrestris on lowering blood lipids through its effect on oxidative defense. In addition, the study of the effect of Tribulus terrestris, saponin, on improving vascular occlusion and sugar reduction by Li et al. (36) and Rodrigues et al. (10) based on its antioxidant properties has been studied.
In this study, the results showed that induction of the disease in animals caused a shift in the balance of PAB to oxidant factors, in which the results were consistent with previous studies (Akbari et al. (37)) showed that H2O2 causes serious damage to the heart cell sarcolemma and causes disorders such as phospholipid peroxidation, decreased ATP, and increased PAB ratio. The results of the present study also showed that the combination of 8 weeks of aerobic exercise and 10 mg of Tribulus terrestris supplementation significantly reduced the antioxidant-antioxidant shift compared to other experimental groups. This combination of aerobic exercise and Tribulus terrestris supplements can be attributed to their role in reducing oxidative stress. So far, no research has been done on the combined effect of regular exercise and the use of thorn plants on inflammatory and antioxidant indicators. However, preliminary research has shown that regular physical activity prevents cardiovascular disease by affecting the ratio of superoxide to peroxide (38). Martinovic et al. (39) also showed that fitness training had created an oxidative imbalance in volleyball women during competitions.
These results are consistent with the findings of de Sousa et al. (6) on the significant effect of exercise compatibility on strengthening oxidative conditions. It has also been reported that drugs such as levastatin can greatly reduce the effects of hydrogen peroxide (2).
Researchers also recommend the simultaneous use of oral herbal supplements due to their antioxidant properties against oxidative stress during strenuous physical activity (40). Therefore, the anti-inflammatory and antioxidant properties of herbal supplements are likely to reduce the toxic effects of hydrogen peroxide. Meanwhile, the effects of Tribulus terrestris extract on improving the variables studied in this study have not been studied. However, the effects of Tribulus terrestris aqueous extract reduce oxidative stress and lipid profile in albino males after induction of necrosis of cardiac tissue by isoproterone (35). Also, the toxicity and oxidative stress caused by the side effects of sodium valproate have been reduced by the use of alcoholic extracts of thistle (11). Tribulus terrestris extract is commonly used to increase energy, clear free radicals, inhibit fat peroxidation, improve the HPG axis function, and enhance physical function (41). Also, its toxic effects have been observed only in very high doses, which were prepared as an alcoholic or aqueous extract (42).
Owing to the limited number of articles examining the simultaneous effects of Tribulus terrestris alcoholic extract and aerobic training, it is very difficult to achieve absolute results. Even in case studies, the type of exercise or method of preparation of the extract, such as aqueous or alcoholic, limits the access to integrated information in this regard. Also, since this study was performed on laboratory rats, limitations such as the inability to accurately control the appetite of rats, the use of Wistar rats to reduce the effects of differences in biochemical responses, possible physiological changes in the laboratory, the effect of oxygenated water and ketamine on blood and enzymatic indices, non-use of a placebo, and gavaging of animals receiving supplements may have affected research results.
5.1. Conclusions
The study's findings show that a combination of aerobic exercise and ten milligrams of Tribulus terrestris could be a good way to reduce the side effects of hydrogen peroxide poisoning. Because the changes continued to be far from the baseline levels of the control-healthy group until complete recovery, longer training periods and higher drug doses should probably be used. In addition to taking Tribulus terrestris before exercise sessions, it is better to use Tribulus terrestris supplementation courses in future studies to strengthen antioxidant defenses. Free radicals and the balance of oxidative defense play an important role in the emergence or control of diseases. Several factors can impair this balance. Poisoning is one of the factors that affect the production of cellular energy by affecting ATP production. In fact, the conflict between defense mechanisms such as the cytochrome C opposition involved in ATP production in mitochondria is one of the reasons for this decrease in cellular energy charge. The use of herbal supplements due to its anti-inflammatory and antioxidant properties is one of the ways to strengthen antioxidant defenses. In addition, regular exercise has been shown to play an important role in increasing the effectiveness of the antioxidant system and in counteracting poisoning. The results of the present study confirm these arguments. The findings of the present study also show that Tribulus terrestris extract in higher doses may have a greater effect on oxidative indicators. Note that these findings have been investigated in the heart tissue of rats and that more general studies are needed to generalize them. Also, the effect of the prescribed amount of this extract on other physiological axes such as HPG needs to be further investigated.