1. Background
Osteoarthritis (OA) is a chronic, multifactorial, and progressive musculoskeletal system disease, which is among the most expensive health problems to treat and rehabilitate (1). As a chronic degenerative disorder, OA is the most common disease of the synovial joints in humans that causes chronic pain, joint stiffness, muscle weakness, decreased range of motion, decreased neuromuscular system efficacy, severe performance disabilities, and reduced social activities. Besides, it negatively affects the quality of life (QoL) and the overall health of patients (2). The World Health Organization (WHO) predicts that, by 2020, osteoarthritis will be the fourth leading cause of disability and frailty (2). People with OA are at increased risk of developing cardiovascular diseases (CVDs) (3), so that 23% of patients with coronary heart disease also have osteoarthritis (4). Although OA is sometimes referred to as a degenerative disease, recent studies have shown that synovial inflammation is an important risk factor in the OA initiation and progression. Therefore, in OA patients, systemic inflammation is a risk factor for CVDs (3). CVDs is the leading cause of death worldwide, and coronary artery disease, particularly myocardial infarction, is the most common heart disease (5). Myocardial infarction causes permanent damage to the heart muscle during the mitochondrial function. Hence, the association between mitochondrial abnormalities and the progression of heart failure is well acknowledged.
There is convincing evidence of an association between mitochondrial dysfunction and myocardial infarction through causing mitochondrial respiratory dysfunction and decreasing the mitochondrial function, that strongly depend on the morphological and structural changes, known as mitochondrial dynamics, and are constantly divided into fusion and fission processes. Fusion proteins (OPA1, mitofusin 1, and MFN2) and fission proteins (DRP1 and FIS1) heavily influence these two opposing processes. Fusion and fission processes are both necessary for cellular metabolism to facilitate the separation of damaged or impaired mitochondria before apoptosis. Impairments in fusion proteins (MFN2, MFN1, and DRP1) causes structural disruption of mitochondria, metabolic disorders of mitochondrial degradation, apoptosis, and (rarely) cell death. Mitochondrial dysfunction not only leads to lipid accumulation but also increases the ROS and cytokines that, in turn, increase the risk of developing the OA. Regulation of mitochondrial dynamic proteins or overextended inhibition of mitochondrial fission reduces mitochondrial dysfunction, which in turn improves the myocardial infarction. Based on the aforementioned, since these proteins regulate the mitochondrial dynamics, they can be targeted to prevent CVDs, including myocardial ischemic heart damage (5). Since there is no definitive cure for OA, physicians mainly intend to relieve the symptoms, primarily pain. Also, since OA is characterized by cartilage loss and bone margin enlargement, patients mostly experience pain, stiffness, and limited range of motion in joints (6).
Since OA is age related, and most of the patients are elderly, special attention should be paid to the length of treatment and health expenditures. Therefore, developing complementary therapies to achieve more desirable outcomes should be prioritized. Among current treatments, ozone therapy can improve the metabolism of knee cartilage tissue due to its anti-inflammatory properties and increase tissue oxygen content (7). Various studies have reported that ozone therapy can reduce inflammatory-related factors and seduce pain in patients with knee OA (8). However, a study that followed patients for three months, reported that ozone therapy had no significant effect on pain relief in OA patients, but could decrease the pain level after six months of administration (9). Since the ability to perform daily everyday activities and reducing the pain are the most important factors to increase the QoL of OA patients, patients with knee osteoarthritis usually appear to have reduced ability to perform their daily activities. Therefore, as a non-invasive and low-cost treatment method, exercise is proposed by health researchers to improve the QoL of OA patients. Regular and aerobic exercise can improve joint diseases by increasing joint metabolism and synovial fluid. Moreover, such exercises have antioxidant and anti-inflammatory effects, which can further improve the QoL of patients (10).
2. Objectives
Several studies have shown that complementary methods are effective in improving or preventing the progressive effects of osteoarthritis as well as to reduce pain and enhance the QoL of these patients, but their mechanism of action is not yet fully understood. Evidence on the interactive effects of aerobic training and ozone therapy on mitochondrial dynamic regulatory proteins in OA patients are not sufficient. Therefore, the present study aimed to investigate the effect of aerobic training and ozone therapy on MFN1 and DRP1 gene expression in the heart tissue of rats with osteoarthritis.
3. Methods
To conduct the present experimental study, 30 rats were purchased and transferred to an animal lab. After a week of adaptation to the laboratory conditions, osteoarthritis was induced to 24 rats through surgery and according to the study of Zhao et al. (11) In this procedure, rats were first anesthetized with ketamine and xylazine, then their knees were completely shaved, and an area of one centimeter was cut at the knee joint.
After removing the skin, the lateral internal ligament of the knee was removed so that the internal meniscus could be seen. Then, through an incomplete incision, a rupture and damage were made to the meniscus, before suturing the area in a sterile manner. The rats were then fed standard food for three weeks and kept in the laboratory setting. Afterward, rats were randomly assigned into four groups of six rats, including (1) osteoarthritis control, (2) training, (3) ozone, and (4) training + ozone. To evaluate the effects of OA on the research variables, the remaining 6 rats were included in the healthy control group.
Before initiating the training program, rats in the aerobic training groups were adapted to a rodent treadmill for one week (three times per week, VO2max 60% - 70%, speed of 16 m/min at 0% inclination for 10 min/day). Briefly, the training program was initiated with a 30-minute run on a treadmill with zero slope and a speed of 16 m/min in the first week, which gradually reached 50 minutes in the eighth week. Warm-up and cool-down stages were considered for 5 m/min at the beginning and end of the training (12).
In the present study, ozone was produced by a low-intensity electric discharge, and its concentration was measured using ultraviolet light at 254 nm. Ozone was injected into the tibiofemoral knee joint at a concentration of 20 μg/mL once a week for three weeks, 21 days after arthrosis of the rats (13). Forty-eight hours after the last training session and ozone therapy, rats were anesthetized with ketamine and xylazine. The heart tissue of rats was then extracted and placed in a nitrogen tank, and after freezing, it was transferred to the laboratory to measure the levels of DRP1 and MFN1 gene expression. The primers of DRP1, MFN1, and GAPDH were designed using Gene Runner software (version 6.5.52). Levels of DRP1 and MFN1 gene expression were measured by real-time PCR, and the relative expression of genes of interest was calculated by the REST software according to the 2-∆∆Ct method. The sequences of DRP1 and MFN1 primers are presented in Table 1.
| Gene Name | Gene ID | Primer Sequences | Tm, °C | PCR Product Length |
|---|---|---|---|---|
| DRP1 | 114114 | Forward: 5’-TGTACTCCCAATTCCATTATCCT-3’ | 56, 57 | 178 |
| Reverse: 5’-CCCTTCCCATCAATACATCCA-3’ | ||||
| MFN1 | 192647 | Forward: 5’-CTCCTGTAATCTTGCCTG-3’ | 52, 56 | 116 |
| Reverse: 5’-ATCGGATCTTTTTTGTTTCAGC-3’ | ||||
| GAPDH | 24383 | Forward: 5’-AGGTCGGAGTCA ACGGATTTGGT- 3’ | 61, 61 | 121 |
| Reverse: 5’-CATGTGGGCCATGAGGTCCACCAC-3’ |
3.1. Statistical Analysis
To assess the normality assumption of the data, the Shapiro-Wilk test was used. Also, one-way ANOVA with Tukey’s post- hoc tests were used to analyze the data (P ≥ 0.05).
4. Results
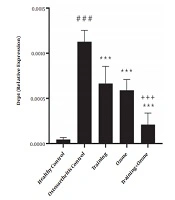
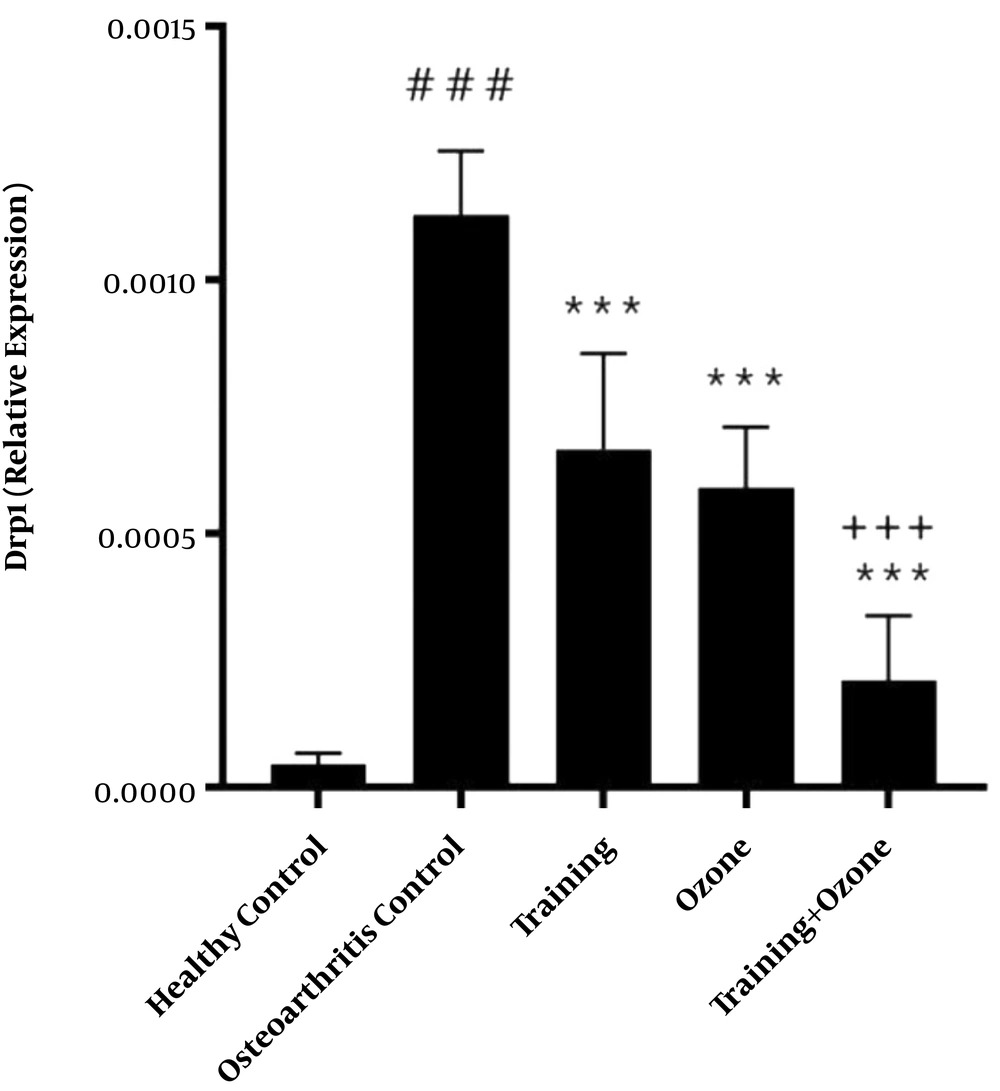
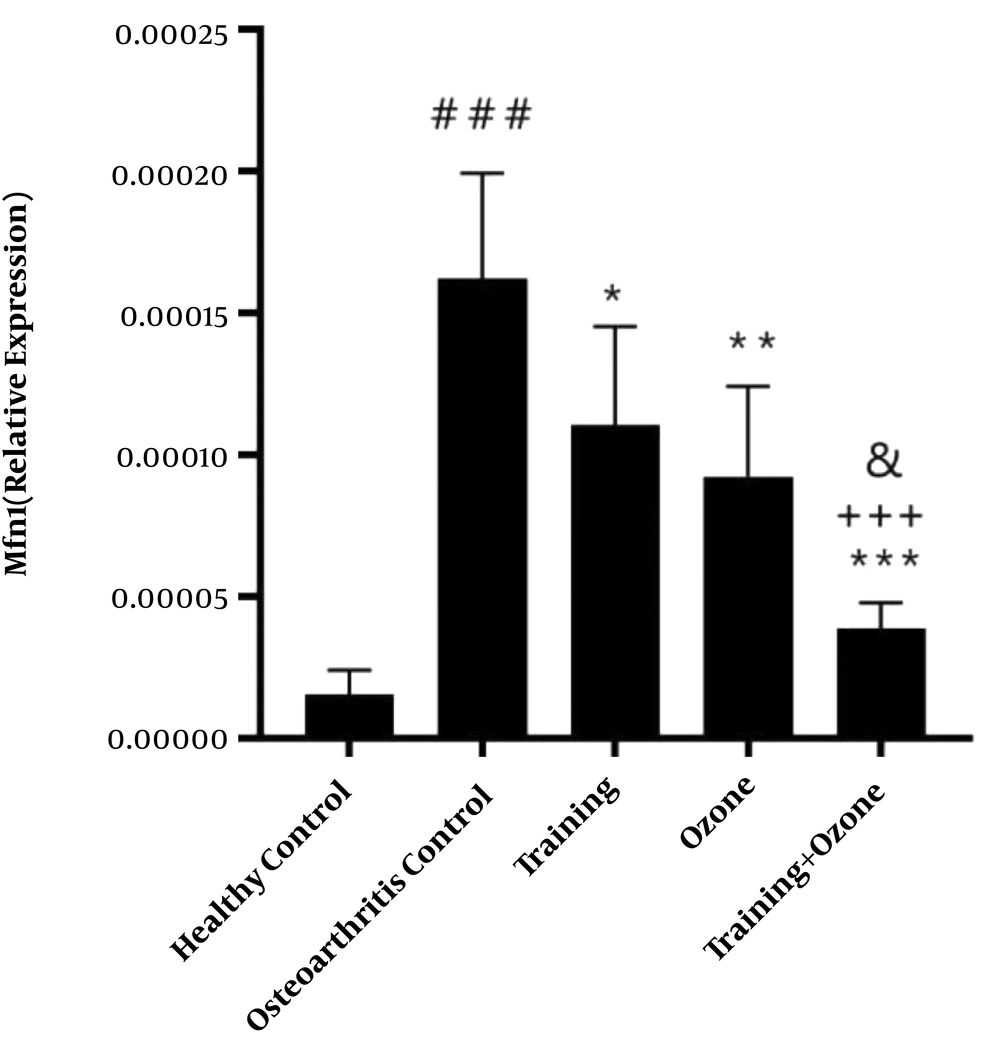
Gene expression levels of DRP1 and MFN1 are presented in Figures 1 and 2, respectively. The one-way ANOVA test showed a significant difference in the gene expression levels of DRP1 (P = 0.001) and MFN1 (P = 0.001) in the five groups of the study.
DRP1 gene expression levels in the five research groups. ###, P ≤ 0.001 significant increase compared to the healthy control group. ***, P ≤ 0.001 significant decrease compared to the osteoarthritis control group. +++, P ≤ 0.001 significant decrease compared to the training and ozone groups.
MFN1 gene expression levels in the five research groups. ###, P ≤ 0.001 significant increase compared to the healthy control group. ***, P ≤ 0.001; **, P ≤ 0.01; *, P ≤ 0.05 Significant decrease compared to the osteoarthritis control group. +++, P ≤ 0.001 significant decrease compared to the training group. &, P ≤ 0.05 significant decrease compared to the ozone group.
The Tukey’s post- hoc test also showed that DRP1 gene expression levels in the osteoarthritis control group were significantly higher than the healthy control group (P = 0.001). However, in the training, ozone, and training + ozone groups, the levels were significantly lower than the control group (P = 0.001). Also, in the training + ozone group, the levels were significantly lower than the training and ozone (P = 0.001) groups (Figure 1).
The Tukey’s post- hoc test showed that the gene expression levels of MFN1 in the osteoarthritis control group were significantly higher than the healthy control group (P = 0.001). However, in the training (P = 0.02), ozone (P = 0.002), and training + ozone (P = 0.001) groups, the levels were significantly lower than the control group. Also, in the training + ozone, the levels were significantly lower than the training (P = 0.001) and ozone groups (P = 0.02) (Figure 2).
5. Discussion
This study showed that the induction of osteoarthritis could significantly increase the DRP1 and MFN1 gene expression levels in the heart tissue of rats. However, eight weeks of aerobic training was associated with the significant decrease of DRP1 and MFN1 gene expression levels in the heart tissue of rats with osteoarthritis. Consistent with the findings of the current study, Jiang et al. (14) showed that aerobic interval training significantly reduced DRP1. Damirchi and Ebadi (5) also reported that six weeks of low-, medium-, and high-intensity interval training was associated with a significant reduction of DRP1, and HIIT was associated with a significant increase of MFN2 in the heart tissue of rats (5). The consistency of the results of this study with the current study can be attributed to the similarity of the study period, the intensity of activity, and using similar tissues. According to the evidence, exercise prevents CVDs through (possibly) risk factors associated with CVDs, improving cardiac physiological growth, increasing antioxidant capacity, and improving mitochondrial function. It seems that through affecting the mechanisms related to the dynamics of mitochondria, exercise positively affects the recovery from heart diseases (15). In this regard, Peyravi et al. (15) showed that eight weeks of interval and continuous training significantly reduced DRP1 gene expression levels and increased MFN2 gene expression levels in the soleus muscle tissue of diabetic rats.
Cardiac reconstruction is a dynamic process that contains a complex regulatory network that includes several biological events (e.g., improvement in mitochondrial function). Regarding the effect of exercise on mitochondrial dynamic regulatory proteins, it has been argued that reactive oxygen species (ROS) play an important regulatory role in this process.
Mitochondrial ROS stimulated by exercise can cause rapid changes in the expression of proteins of fusion and mitochondrial fission. Studies indicated that oxidative stress causes an imbalance in mitochondrial fission. However, it worth noting that the role of ROS in mitochondrial fission and conversion to decomposed units depends on the concentration and duration of exposure. Accordingly, strenuous exercise is associated with increased levels of different ROSs, thereby affecting fission and fusion proteins (5). Also, the results of the present study showed that ozone therapy for eight weeks significantly reduced the gene expression levels of DRP1 and MFN1 in the heart tissue of rats with osteoarthritis. In line with the findings of the present study, several studies have mentioned the therapeutic effects of ozone therapy on the heart tissue (16-19). Ozone therapy is a new treatment that is becoming an effective therapeutic option for musculoskeletal pain. Ozone is a highly soluble, oxidizing gas that, in contact with biological water, can make ROS-induced products possible. These substances react with white blood cells initiating the production of some other products, including inflammatory cytokines and red blood cells, which in turn increase oxygen delivery to the tissue (20). It’s believed that the ozone analgesic mechanism triggers by the stimulation of the antinociceptive apparatus through endogenous opioids and serotonin, which may increase the pain threshold. Besides, some studies have suggested that the anti-inflammatory properties of ozone may be associated with reduced edema and joint swelling and pressures on the nerve structures, which in turn may reduce the pain (20). Ozone can increase tissue oxygenation by several mechanisms following increased vascularization (following new angiogenesis), which in turn results in improved local tissue nutrition and inhibitory capacity of inflammatory metabolites (20).
This study showed that eight weeks of simultaneous aerobic training and ozone therapy was associated with a significant decrease in the gene expression levels of DRP1 and MFN1 in the heart tissue of rats with osteoarthritis. Simultaneous training and ozone therapy was also more effective than sole training and ozone therapy, in terms of reducing the levels of DRP1 and MFN1 gene expression in the heart tissue of rats with osteoarthritis. In line with the protective effect of exercise and ozone therapy, Asadi et al. (21) reported that aerobic training and ozone therapy alone increased interleukin-10, as an anti-inflammatory cytokine, and decreased TNF-α in the cartilage tissue of rats with knee osteoarthritis. Therefore, it can be concluded that regular and aerobic exercise can improve joint disease by increasing joint metabolism, synovial fluid, as well as antioxidant and anti-inflammatory effects. Since patients with knee osteoarthritis experience decreased ability to perform everyday activities, the findings of the present study showed that aerobic training, as a non-invasive and low-cost treatment method, and ozone therapy have similar effects on improving mitochondrial dynamic regulatory proteins such as MFN1 and DRP1 in the heart tissue of rats with osteoarthritis. The current study had limitations, including not measuring the levels of MFN1 and DRP1 protein by the ELISA and Western blot methods. Therefore, to confirm the findings of the present study, it is recommended to investigate the effects of aerobic training at different intensities along with ozone therapy on protein levels indicating mitochondrial biogenesis such as MFN1, DRP1, MFN2, and PGC1α in the heart tissue of rats with osteoarthritis in future studies.
5.1. Conclusions
Although eight weeks of training and ozone therapy was associated with improved gene expression levels of MFN1 and DRP1 in the heart tissue of rats with osteoarthritis, the concurrent implementation of training and ozone therapy results in better outcomes on improving the gene expression levels of MFN1 and DRP1 than sole providing of either one.