1. Background
Aging is associated with a gradual reduction of functional abilities, which results in limitations in performing daily living activities (1, 2). Simultaneous decrease of both cardiorespiratory fitness (3) and peak muscular power output (4) with aging is well-documented, which negatively influence independence in daily living (5) and increase the risk of morbidities (6). In other words, reduced physiological capacity due to aging is the main obstacle to attain heightened well-being span, diminished morbidity, and optimal life span (7). Moreover, aging is accompanied by multiple changes in the body, mainly due to hormonal changes (8, 9).
Testosterone is well accepted as a strong anabolic hormone in males, as illustrated by the efficacy of pharmacologically administered testosterone, clinical impacts in hypo-gonadal subjects, and a stimulatory impact on anabolic pathways such as mTOR (10-12). Several longitudinal surveys reported that after the age of 40, each year of life is accompanied by 0.4 to 2.6% reduction in the concentration of testosterone (13, 14). In the seventh decade of life, it increases faster (14). In contrast, it is demonstrated that the concentration of sex hormone-binding globulin enhances with age, which results in a faster decline of the biologically active portion of free testosterone (13). A reduced level of testosterone is associated with muscle atrophy and reduced functional capability (15).
Insulin-like growth factor-1 (IGF-1) is a major anabolic factor in the human body (16). IGF-1 is firmly associated with muscle growth, skeletal muscle system preservation, metabolism, and muscle power (17, 18). However, its secretion declines with aging (19, 20), which in turn increases susceptibility to illness and dependence in daily activities (21-23). Nevertheless, aged-individuals skeletal muscle might reconstruct as a reaction to physical activity by heightening the IGF-1 supply and developing the heavy chain of myosin (24). In addition, physical activity has lately been displayed to considerably contribute to preserving the functional capability of older adults (25, 26). Physical activity recommendations for adults and the elderly have emphasized that flexibility, aerobic, strength, and resistance exercises should be part of all training programs (27). Few favorable comprehensive exercise prescriptions are defined for older adults. Based on what was mentioned before, efficient exercise programs not only are associated with decreased incidence of chronic diseases but also increase the physical and psychological function of the elderly, all of which result in increased life expectance.
Aquatic exercise is common among aged-individuals due to its weight-bearing pressures decline on the skeletal joints and therapeutic advantages in situations of orthopedic disorders (28, 29). Furthermore, since the air density of water is 700 times higher, aquatic exercise is a conceivable method to enhance cardiovascular fitness (30), which promotes a decline of the effect on the joints, and partial weight support is supplied by water buoyancy (2).
2. Objectives
Thus, while aquatic exercises may be appropriate for the elderly, the impacts of usual water exercise on serum testosterone and IGF-1 levels of elderly men are little known. Therefore, the current study aimed to assess the effects of water exercise on serum testosterone and IGF-1 concentration for elderly men based on the hopeful nature of water exercise. In addition, the effectiveness of an aquatic exercise program was also evaluated compared to a land-based exercise program because the literature on this field is limited.
3. Methods
In this semi-experimental study with pre- and post-test analyses, 24 men aged 60 - 75 years were recruited by sending an advertisement to the office of pensioners and recreational sports centers of Mashhad. Before including participants, first, they were medically examined. Moreover, they were asked to fill two questionnaires on medical history and physical activity. Then, those who had the inclusion criteria were asked to fill the written informed consent. All participants were informed that participation is voluntary, and they can withdraw any time from the study (Table 1).
| Variables | WBC (n = 10) | LBC (N = 10) | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Age, y | 64.1 ± 5.4 | - | 63.8 ± 4.3 | - |
| Height, cm | 170.1 ± 5.6 | - | 170.8 ± 6.6 | - |
| Body mass, kg | 72.4 ± 8.4 | 71.2 ± 8.7 | 73.7 ± 11.0 | 72.9 ± 11.3 |
| BMI, kg/m2 | 25.2 ± 2.4 | 24.74 ± 2.9 | 25.0 ± 3.7 | 24.8 ± 3.9 |
Abbreviation: BMI, body mass index; LBC, land-based cycling group; WBC, water-based cycling group.
aValues are expressed as mean ± SD.
Exclusion criteria were as follows cardiopulmonary illness, endocrine disorders (e.g., type 2 diabetes, metabolic syndrome), using hormone supplementation, infection, physical limitation or neuromuscular disability, participation in other physical exercise programs during the past three months, a history of smoking and/or any drug that affects the level of testosterone and IGF-1.
Four participants (out of 24) had at least one of the exclusion criteria and were excluded from the study. After measuring baseline body composition and taking the blood samples, participants were randomly assigned into two groups of water-based cycling (WBC) and land-based cycling (LBC), each with 10 members. The subjects have been asked to avoid to participate in any physical exercise activity during the study.
All procedures performed in studies involving human participants should be in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The present study was approved by the Ferdowsi University of Mashhad, Iran (code: IR.MUMS.REC.1397.203), and was conducted in accordance with the Helsinki Protocol.
3.1. Interventions
All sessions were instructed by a fitness instructor under the supervision of the researchers, for both exercise training protocols. Both groups received an 8-week cycling program for 3 sessions per week.
3.1.1. Water Exercise Group Protocol
Subjects performed cycling in the water on a stationary bike with an intensity of 60% to 70% of the maximum heart rate (HRmax). The HRmax was calculated based on the formula (age-220) and participants were controlled with a polar heart rate monitor (Polar Electro, Finland) placed on the chest of the subjects. The duration of each training session was 30 minutes in the first week, then increased by 2 minutes each week.
3.1.2. Land Exercise Group Protocol
Subjects cycled on a stationary bike with an intensity of 60% to 70% of HRmax, which was calculated based on the formula (age-220) and its control was performed with a polar heart rate monitor (Polar Electro, Finland). The duration of each training session was 30 minutes in the first week, which increased by 2 minutes per week.
3.2. Procedure of Data Collection
3.2.1. Anthropometric variables
The study administrator, calculated body composition parameters at baseline and 8 weeks. Height (cm) of all participants were measured utilizing a digital stadiometer (Seca, Germany) to the nearest 0.1 cm. To assess body mass and body mass index (BMI), we utilized an Inbody X-CONTACT 357S Body Composition Analyzer (South Korea).
3.2.2. Analyzing Testosterone and IGF-1 Serum Levels
The participants were submitted to blood collection between 8 a.m. to 10 a.m., after a 12-h fast to measure testosterone and IGF-1 serum levels in a clinical analytic laboratory. Testosterone and IGF-1 were analyzed by the ELISA Kit (Monobind and LDN companies, respectively) in accordance with the manufacturer guidelines. At the end of the study, blood sampling were done similar to the early study for both groups.
3.3. Statistical Analysis
Statistical analyses were performed using SPSS version 20 (IBM, Armonk, NY), and the results are expressed as mean and standard deviation. The normal distribution of data was evaluated using the Shapiro-Wilk test. Changes in variables from the baseline to the end of intervention within the groups were analyzed by the Paired sample t-test. An independent sample t-test was employed for intergroup comparisons. Statistical significance was considered when the P value < 0.05.
4. Results
The baseline characteristics of the participants are described in Table 1. Anthropometric variables were similar between the two groups at baseline (P > 0.05).
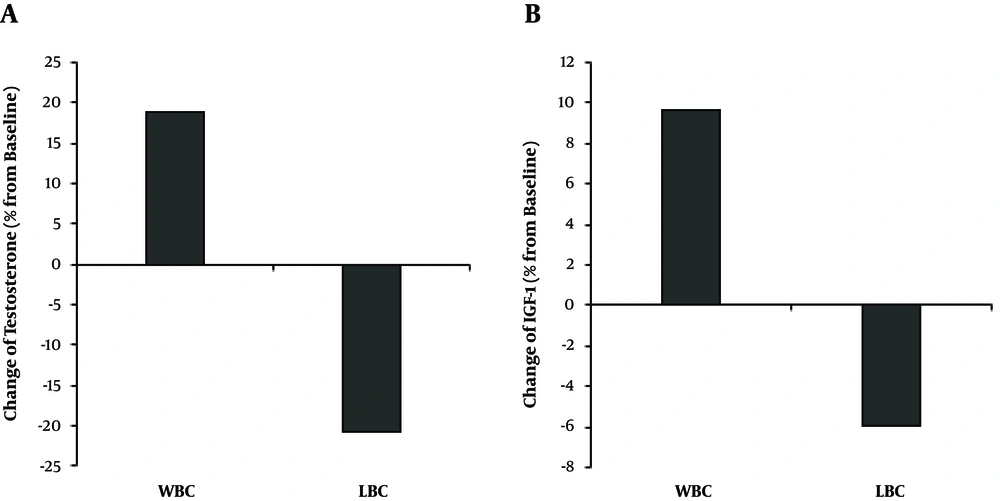
After providing the intervention, the serum testosterone level was significantly increased in the WBC group (18.75%, P = 0.010). However, it was significantly reduced in the LBC group (20.69%, P = 0.042). The intra-group comparison showed that in the WBC group, testosterone was significantly different (P < 0.05) from the LBC group (Table 2 and Figure 1).
Abbreviation: WBC, water-based cycling group; LBC, land-based cycling group.
aValues are expressed as mean ± SD.
bSignificance differences in compared with pre-test value.
The serum IGF-1 level was significantly increased in the WBC group (9.69%, P = 0.005), though, it for the LBC group, it was almost untouched after providing the intervention (-5.9%, P = 0.555). The intra-group comparison showed that in the WBC group, IGF-1 was not significantly different between the two groups (P > 0.05) (Table 2 and Figure 1).
5. Discussion
Based on the findings, elderly men who participated in the 8-week WBC program had higher levels of testosterone after fulfilling the program. On the other hand, in the LBC group, the serum testosterone level was significantly decreased (Table 2 and Figure 1). To our knowledge, this is the first study to compare the effects of water versus the LBC program on the hormonal changes in elderly men.
The mechanism for enhancing serum testosterone concentrations throughout short-term practice is contentious and could be attributed to the enhanced production of testosterone, reduced clearance of testosterone, and/or hemoconcentration (31). According to the literature, sex hormone-binding globulin (SHBG) is a high-affinity testosterone-binding glycoprotein, that binds to about 50% of the circulating testosterone. Although, it has delayed effects on testosterone hepatic clearance (32-34). Zmuda et al. (35) reported that alteration in SHBG levels throughout exercise training was positively associated with testosterone levels. Besides, the authors noted that the temporal relation among variations in SHBG and testosterone was noticeable. Hence, they argued that enhanced SHBG may affect the levels of testosterone during exercise (35). Therefore, in the current study, the increased level of testosterone in the WBC group can be attributed to enhanced SHBG. It worth noting that, in the present study, the participants’ SHBG concentrations have not been measured.
The significant decrease in circulating testosterone levels following LBC is somehow different from the results of some of the previous studies (36-38). In contrast, Lovell et al. (39) reported that thrice-weekly exercise training in 16 weeks could not enhance resting total testosterone or free-testosterone in elderly subjects. Few studies are conducted on the response of sex hormones to exercise in aging men. These different results can be attributed to the participants’ training status, which in turn affects the outcomes of subsequent exercise training interventions.
Probably the intensity of the training program has influenced the exercise-induced testosterone response. Lovell et al. (39) trained subjects for 75 - 135 min per week, whilst the training program of Khoo et al. (38) lasted for 90 - 150 min per week (low volume) and 200 - 300 min per week (high volume), and the authors reported that only in the high-volume group the total testosterone level was increased. In the current research, the LBC group was trained for 90 - 160 min per week, to achieve an exercise-induced testosterone response in male elderly, the training volume should be more than 150 min per week. However, further investigations are required to evaluate this threshold.
Based on the results, an 8-week WBC program could increase the IGF-1. On the other hand, in the LBC group, the IGF-1 level remained unchanged (Table 2 and Figure 1). This finding can be attributed to applying lower levels of exercise intensity than what was mentioned in the protocol. To the best of our knowledge, this is the first study comparing the effects of water versus the LBC program on the IGF-1 changes in male elderly. In the following, the findings of the present study are compared to other related studies. Vale et al. (40) reported increased IGF-1 in the land-resistance training group, although the IGF-1 did not alter in the water-resistance training group, which is in contrast to the findings of the present study. Furthermore, Orsatti et al. (41) found a significant enhance in the levels of IGF-1 and upper and lower limb strength in aged-postmenopausal submitted to land resistance training of 60% - 80% one-repetition maximum (3 sets of 8 - 12 repetition) for 16 weeks (3 times/week) compared to a control group. Nevertheless, Vale et al. (42) found no significant variation in the IGF-1 throughout a 12-week aerobic aquatic program, which is in agreement with our results. But Ay and Yurtkuran (43), in a study on 41 postmenopausal women yielded to low-intensity 6-month controlled aerobic aquatic exercises, according to the Borg scale, reported a significant rise in IGF-1. It can be argued that the type of exercise training can affect the IGF-1 response. Two review studies have reported that resistance exercises was more effective in inducing IGF-1 increasing compared to aerobic exercises (44, 45). However, alteration of IGF-1 in the WBC group may be caused by the dual effects of buoyancy and resistance created in this environment, which requires high levels of energy consumption.
IGF-1, the main peptide hormone with biologic activity like insulin, is an essential mediator of various anabolic influences of growth hormone (GH). The liver is the main source of IGF-1 synthesis, which releases as an endocrine hormone into the systemic circulation to be attached to one or more binding proteins. Additionally, IGF-1 is produced in body tissues where it applies its paracrine activities (46).
5.1. Conclusions
This study demonstrated that WBC could increase the serum level of testosterone and IGF-1 in the male elderly. Nevertheless, LBC training couldn’t change the investigated hormones. It can be reported that WBC protocol can stimulate anabolic effects in older adults. Therefore, this research opens perspectives for additional study that may include other kinds of water training and control of the serum concentrations of other hormones, principally related-anabolic hormones, which change after aging.