1. Background
The word apoptosis is a Greek word meaning the fall of a deciduous tree. In 1972, when Kerr et al. first observed the difference between necrosis and apoptosis, they did not think that their exploratory phenomenon would one day be at the forefront of anti-cancer studies (1). This process occurs with the irreversible fragmentation of DNA and the formation of apoptotic bodies attached to the membrane, which results in the cleavage of vital cell proteins (2, 3). There is some evidence that exercise may affect a number of apoptotic signaling pathways in the skeletal muscle (4). In response to apoptotic stimuli, a range of external and internal signals regulate the expression of genes that control the onset of apoptosis. In the internal pathway, genes express pro-apoptotic proteins (including Bax , Fas, P53, etc.) and anti-apoptotic proteins such as (Bcl-2 and Bcl-XL), and its outcome for the cell (death vs. survival) depends on the proportion of expressed genes. Bcl-2 is one of the most well-known apoptotic inhibitory proteins that inhibits the release of cytochrome C from mitochondria. In the external pathway (or death receptors) this pathway begins following ligation of the TNF receptor family and leads to activation of caspase-8 and subsequently caspase-3. Caspases are proteases that are synthesized as inactive zymogenes and undergo proteolytic failure during apoptosis. Further research has shown that shifting the Bax protein to the mitochondria and placing it inside the outer membrane releases other apoptotic agents (such as cytochrome c) from the mitochondrial interstitial space (5). However, Bcl2 protein counteracts the pre-apoptotic activity of Bax protein and preserves the integrity of the mitochondrial membrane (6-8). Finally, apoptotic messages are aligned in the activation of commonly effective caspases, including caspase-3, and cause possible cell destruction (2, 6, 8, 9). Therefore, researchers are always looking for appropriate strategies to prevent apoptosis and various muscle diseases associated with it. In the last decade, the effect of exercise on apoptosis has been of interest to researchers in the field of exercise (9). In this regard, a number of researchers have suggested that moderate-intensity continuous exercise may reduce apoptosis in various tissues (6, 8, 9). Unlike exercise, however, a session of strenuous exercise for up to 48 hours can accelerate the process of apoptosis (10, 11). Kim et al. (2010) reported that aerobic training decreased Bax protein expression, Bax/Bcl-2 ratio, and caspase-3 activity in rats’ soleus muscle tissue and increased Bcl-2 protein gene expression (12). Song et al. (2006) also noted that aerobic training increased Bcl-2 levels while decreasing caspase-3 activity, Bax, and Bax/Bcl-2 ratio in the calf muscle and soleus muscle of rats (7). However, contrary to the results of these studies, Liu et al. (2013) showed that nine weeks of endurance training significantly increased Bax and Bax/Bcl2 ratio in the skeletal muscle of rats (11). It should be noted that differences in the intensity and duration of aerobic training used in different studies have led to conflicting results in skeletal muscle apoptosis. Due to the better effect of interval training and some indicators such as aerobic capacity and increasing use of these exercises by athletes and ordinary people and even some patients, the effect of this type of training is a topic that can attract the minds of sports researchers. Considering the use of medicinal plants with therapeutic and antioxidant properties, one can refer to the saffron plant. Saffron has several compounds, including crocin, crocetin, and safranal. Among its active components, crocin is the main cause of its various pharmacological activities (13). Crocin is one of the most important carotenoids in saffron and the causative agent of saffron dye. Among the properties of crocin, we can mention its anti-atherogenic and anti-clotting, as well as the nervous system and neuronal protective effects (14). Crocin has been shown to reduce doxorubicin-induced cardiac toxicity by negatively regulating inflammatory and apoptotic pathways in rats (15). Other suggested mechanisms for the antitumor effects of saffron compounds such as crocin include inhibition of nucleic acid and free radical chain reactions, the effect of carotenoids on topoisomerase II, and induction of apoptosis (16).
2. Objectives
Due to the contradictory results of research on the effect of HIIT on apoptotic indices and the effect of HIIT alone and in combination with crocin consumption on apoptotic indices, it is necessary to find an effective non-pharmacological solution. In view of the above, the current study aimed to investigate the effect of eight weeks of HIIT and crocin supplement on the expression of Bax, Bcl-2, and caspase-3 genes in the soleus muscle tissue of male rats exposed to doxorubicin.
3. Methods
In this experimental study, 60 male Wistar rats weighting approximately 220 ± 20 grams (Table 1) were purchased and transferred to the animal laboratory in standard conditions. The animals went through an adaptation phase for seven days. Then they were divided into five groups: 1) control, 2) training, 3) crocin, 4) training + crocin, and 5) healthy control. Groups 1 to 4 received 2 mg/kg doxorubicin (Belgian company Ebeve) weekly by intraperitoneal injection for seven weeks (17). Groups 3 and 4 received 10 mg/kg of crocin (Sigma-Aldrich Company, USA) via oral gavage daily (18), and groups 2 and 4 performed high-intensity interval training, including a combination of interval repetitions with an intensity of 80 to 90% of maximum speed and low intensity with 30 to 40% of maximum speed at two-minute intervals for eight weeks and every week for five days. After 48 hours from the last training session, the rats were anesthetized by injection of ketamine (80 mg/kg), and xylazine (10 mg/kg) (19) and their soleus muscle was extracted, and then, the sample was placed in a cryotube and kept in liquid nitrogen at -70 ° C to check gene expression of Bax, Bcl-2 and caspase-3 proteins. Real time PCR technique was used to measure the expression of these genes. First, primer design was performed, and then total RNA was extracted from tissues and converted to cDNA. The cDNA was then proliferated by PCR and examined for the expression of the mentioned genes. The sequence of primers can be seen in Table 2.
| Group | Body Weight (gr) | Soleus Muscle Weight (gr) | Muscle Weight-to-Body Weight Ratio of Soleus |
|---|---|---|---|
| Healthy control | 289.25 ± 41.34 | 0.113 ± 0.015 | 0.00039067 |
| Doxorbicin- control | 285.50 ± 30.53 | 0.112 ± 0.024 | 0.00039229 |
| Doxorbicin – Training | 239.50 ± 23.07 | 0.142 ± 0.020 | 0.0005929 |
| Doxorbicin-Crocin | 270.25 ± 35.22 | 0.124 ± 0.016 | 0.00045883 |
| Doxorbicin - Training-Crocin | 234.50 ± 22.38 | 0.154 ± 0.027 | 0.00065672 |
| Gene | Forward Primer 5' → 3' | Reverse Primer 5' → 3' |
|---|---|---|
| Gap | AAG TTC AAC GGC ACA GTC AAG G | CAT ACT CAG CAC CAG CAT CAC C |
| Bax | GCAAACTGGTGCTCAAGG | CAGCCACAAAGATGGTCA |
| Bcl2 | GAGTGGGATACTGGAGATGAAG | TGGTAGCGACGAGAGAAGTC |
| Casp3 | AAGTGATGGAGATGAAGGAGT | CAGGCGTGAATGATGAAGAGT |
3.1. High Intensity Interval Training Protocol
The main training protocol included 2-minute alternations of running on a treadmill with a zero percent slope for eight weeks and five days every week and one session per day. It should be noted that before the main training, warm up was performed for 5 minutes with an intensity of 40 to 50% of the maximum speed (16 to 20 meters per minute), and after the main training, cooling was performed for 5 minutes with an intensity of 30 to 40% of the maximum speed (12 to 16 meters per minute) on the treadmill. The main training included performing two 2-minute alternations with an intensity of 80% of the maximum speed (32 m/min) in the first week, four 2-minute alternations with 85% of the maximum speed (34 m/min) in the second week, and six 2-minute alternations with an intensity of 90% of the maximum speed (36 meters per minute) from the beginning of the third week to the end of the period. From the beginning of the fourth week to the end of the period, eight high-intensity 2-minute alternations were performed. Low-intensity alternations consisted of 2-minute alternations with an intensity of 40% of the maximum speed (16 m/min) from the first week to the end of the third week and 30% of the maximum speed (12 m/min) from the beginning of the fourth week to the end of the training period (20, 21).
3.2. Data Analysis Procedure
The data were represented in text, figures, and tables as mean and std. Two-way analysis of variance (HIIT × Crocin) was used to analyze the statistical differences among the groups to verify the main and interaction effects. The significance level was considered as less than 0.05 (Figures 1 to 3).
4. Results
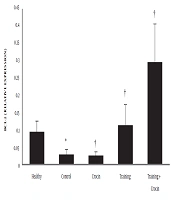
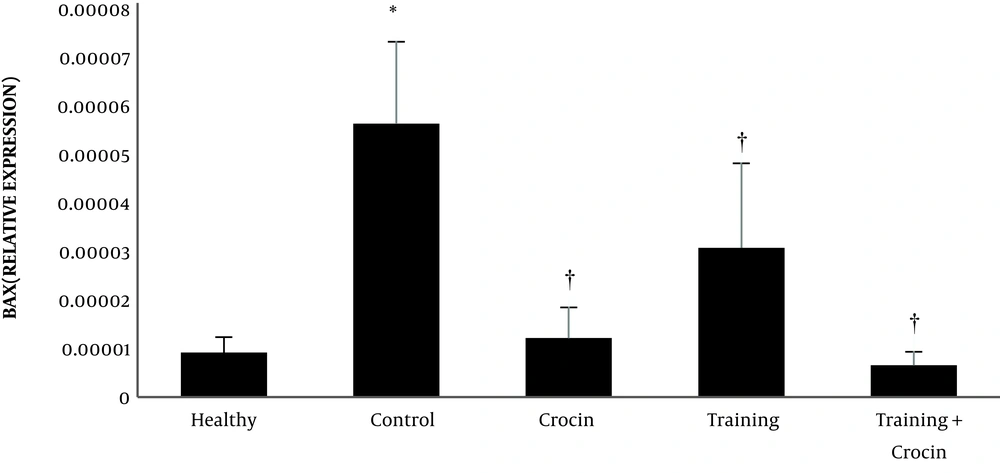
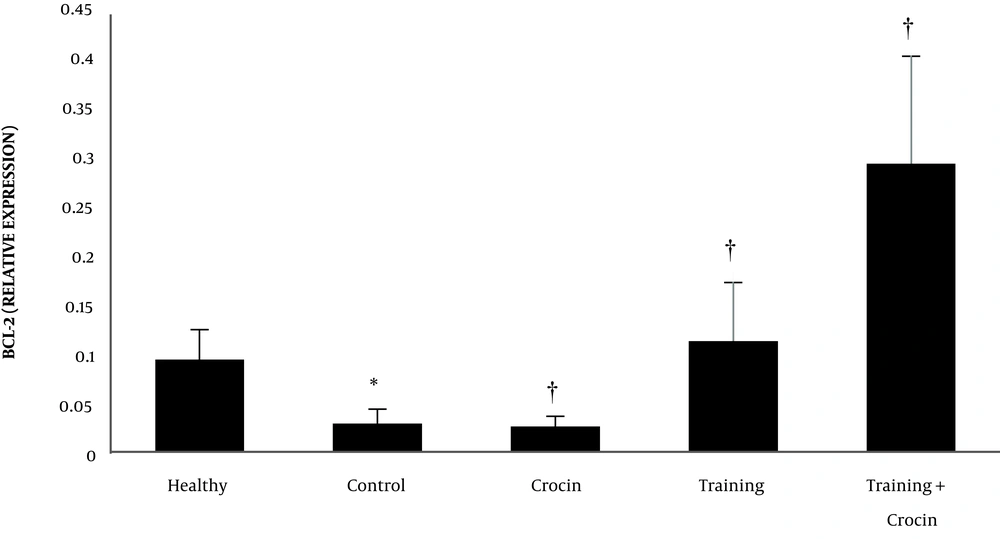
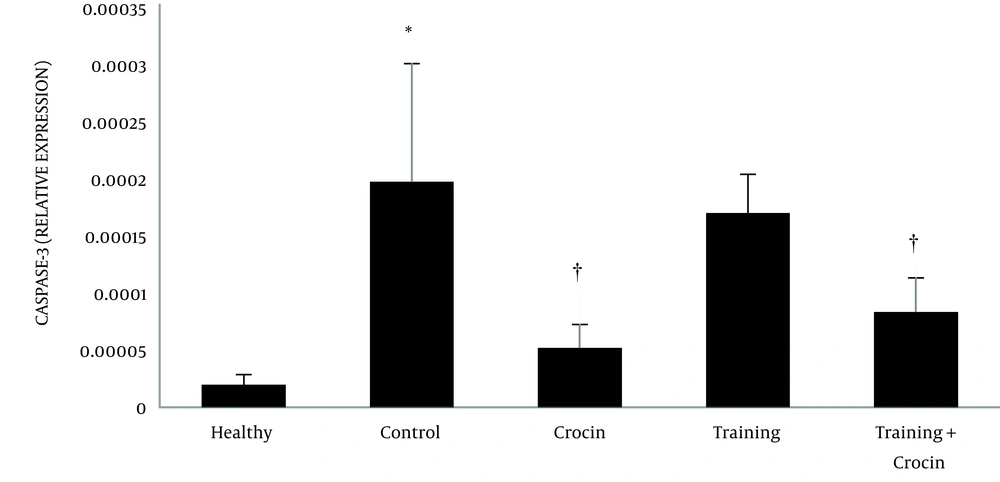
The results of the independent samples t-test showed that Bax (P = 0.001) and caspase-3 (P = 0.001) levels in the Doxorbicin control group were significantly higher than the healthy control group. However, Bcl-2 levels in the Doxorbicin control group were significantly lower than the healthy control group (P = 0.001).
Training (P = 0.004, F = 7.10 and effect size 0.38) and crocin consumption (P = 0.001, F = 49.03 and effect size 0.68) had a significant effect on reducing Bax gene expression. In addition, the interactive effects of training and crocin consumption were significant in reducing Bax gene expression (P = 0.02, F = 6.28 and effect size 0.21). Crocin consumption (P = 0.001, F = 26.63 and effect size 0.53) had a significant effect on reducing caspase-3 gene expression. However, training (P = 0.92, F = 0.07 and size effect of 0.006) had no significant effect on the reduction of caspase-3 gene expression. Furthermore, the interactive effects of training and crocin consumption were not significant in reducing caspase-3 gene expression (P = 0.21, F = 1.61 and effect size 0.06).
Training (P = 0.001, F = 26.03 and effect size 0.69) and crocin consumption (P = 0.002, F = 12.44 and effect size 0.35) had a significant effect on increasing Bcl-2 gene expression. Also, the interactive effects of training and crocin consumption were significant in increasing Bcl-2 gene expression (P = 0.001, F = 13.28 and effect size 0.36).
5. Discussion
The findings of the present study showed that the effect of HIIT with crocin extract caused a significant increase in Bcl-2 and a significant decrease in Bax in the soleus muscle of male rats exposed to doxorubicin. Various studies have been done in this field, some of which are in line with the present research and some of which are inconsistent. Ghahramani et al. (22) concluded that interval training through expression of Bcl-2 and Bax genes results in the reduction of apoptosis in cardiomyocytes after myocardial infarction, the amount of which depends on the intensity of training. In addition, low-intensity interval training has a greater effect than high-intensity interval training. These findings are consistent with the results of the present study in the expression of the Bcl-2 gene and inconsistent in the expression of the Bax gene. Yaghoobpour Yekani et al. (23) showed that high-intensity training significantly increased Bax gene expression, while it had no significant effect on Bcl-2 gene expression, which is inconsistent with the present study. They also showed that high intensity interval training increased the Bax/Bcl-2 ratio compared to moderate-intensity endurance training, which is inconsistent with the present study. Siahkohian et al. (24) showed that Bcl-2 gene expression in the training group was significantly higher than the control group and the Bax to Bcl-2 ratio in the exercise group was significantly lower than the control group. These findings are consistent with Bcl-2 gene expression and inconsistent with Bax to Bcl-2 ratio. However, Bax gene expression was not significantly different between the two groups, which is consistent with the present study. Also, in line with the results of this study, Hasani et al. (25) showed that 8 weeks of HIIT reduced Bax brain levels in elderly female rats and significantly increased Bcl-2 brain levels in training group rats. Farzanegi et al. (26) showed that eight weeks of swimming, garlic extract consumption, and combined intervention significantly increased Bcl-2 and significantly decreased Bax in elderly rats with chronic kidney disease, which was consistent with Bcl-2 gene expression and inconsistent with Bax expression. Heydari et al. (27) found that 8 weeks of resistance training and crocin consumption at doses of 12.5 and 25 mg/kg had a significant effect on catalase but only combination with 25 mg/kg caused a significant increase in tissue glutathione peroxidase. All rats were poisoned with nandrolone. Akbari et al. (28) found that six weeks of aerobic exercise in water and crocin supplementation significantly reduced caspase-3 expression and apoptosis in the heart tissue of male rats poisoned with H202, which is inconsistent with the present study because it can be similar to aerobic exercise. Moradi et al. (29) performed a study on the anti-apoptotic effect of interval and continuous training and crocin on muscle tissue of rats with type II diabetes due to a high-fat diet. It was found that eight weeks of interval training with an intensity of 80 to 85% and continuous training with an intensity of 50 to 55% of maximum speed on the treadmill and consumption of 25 mg/kg of crocin per day increased BCL2 and decreased BAX and P53. The results are consistent with the present study. Physical activity has the potential to modulate cell proliferation and death through cytokines, hormones, growth factors, and metabolic pathways (30). Courtney et al. (31) showed that exercise is a protective factor against caspase-independent apoptosis in aging skeletal muscle by reducing the transfer of EndoG and AIF to the nucleosome. EndoG is a caspase-independent protease that causes DNA fragmentation (32). The findings of the present study also showed that doxorubicin induction was associated with a significant decrease in Bcl-2 protein and a significant increase in Bax protein, wherein regular, high-intensity interval training (of running) along with crocin extract reversed this ratio, i.e., it was associated with a significant increase in Bcl-2 protein and a significant decrease in Bax protein, thereby increasing cell survival. Consistent with the findings of the present study that training and training with crocin did not affect caspase-3 gene expression in the soleus muscle of male rats, Siu et al. reported no difference between the training and control groups in caspase-3 activity after eight weeks of training on the treadmill (32). According to McMillan et al., the absence of a decrease in caspase-3 gene expression activity due to exercise may be the result of a significant decrease in strong levels of caspase-3 inhibitor (XIAP) and an increase in smac levels (XIAP inhibitor) (8). It is also possible that elevated plasma TNF-α and IL-6 mediate direct activation of caspase-3 via the external pathway and preserve caspase-3 (9). Caspase-3 plays an important role in changing the condition of skeletal muscle. Maintaining caspase-3 activity after exercise may be required for cellular functions such as physiological adaptations and differentiation of satellite cells (8). Several mechanisms have been proposed for the protective effects of exercise on muscle apoptosis, including direct changes in the expression of apoptotic proteins, decreased release of mitochondrial apoptogenic agents, and changes in the production of reactive oxygen species (ROS) and antioxidant status (9).
In conclusion, it appears that high-intensity interval training and crocin consumption alone can improve the doxorubicin-induced apoptosis process. However, simultaneous high-intensity interval training with crocin extract has more favorable effects on the doxorubicin-induced apoptosis process than either alone.
Therefore, exercise, the use of crocin supplements, and a combination of two methods can be considered as effective non-pharmacological treatments to reduce muscle damage and protect muscle tissue against damage caused by the side effects of chemotherapeutic drugs. However, given the limitations of this study, such as using the TUNEL and Western blot method, more research is needed to identify the effects of exercise training and crocin on indices of muscle apoptosis.