1. Background
Tissue engineering is an interdisciplinary science branch that combines engineering and life science and uses new materials for the restoration of damaged tissues to improve their function (1, 2). Peripheral nervous system injuries are common and currently have no definitive treatment (3). About 2.8% of patients with trauma suffer from these injuries (4). Peripheral nerve injury can be due to chemical, thermal, mechanical, and pathological damages (5, 6). Nervous system repair is a complex biological phenomenon. When the damage is mild, the nerve can repair itself (7). In extreme injuries that result in a longer nerve gap, repairing the damaged nerve requires surgery, and the use of autologous nerve autograft for reconstruction of the nerve is considered a clinical gold standard (8, 9).
Although using an autograft is considered to be the best strategy for the reconstruction of injured nerves, it has some disadvantages, such as the formation of neuromas and limited availability of donor tissue. Many studies have been conducted to find an appropriate scaffold using synthetic and biological materials (10). Therefore, tissue engineering neural grafts are an appropriate replacement for autologous nerve transplantation for the regeneration of peripheral nerve injuries. Some of these grafts have been used to fill the peripheral nerve gap in animal models, resulting in optimal nerve tissue regeneration and functional improvement (11). Acellular nerve graft, in addition to peripheral nerve repair, can be used as a model for investigating the interactions of the extracellular matrix of the peripheral nervous system. This type of grafts acts as a physical scaffold and provides an appropriate condition for growth, reproduction, and cellular migration (12).
Mesenchymal stem cells (MSCs) have the potential to become nerve cells, osteoblasts, chondrocytes, and other cells (13). Wharton’s jelly is a gelatinous cord tissue that has stromal cells. wharton’s jelly stem cells (WJSCs) can be separated from umbilical cords and differentiate into different cell types (14). These cells are immunosuppressive and are more active than adult mesenchymal stem cells (15). Cell-based therapies have an important role in the regeneration of peripheral nerves. However, cells have been shown to decrease and dead at the beginning hours following the transplantation when seeded on the scaffolds due to the harmful microenvironment of the injured site.
To overcome this problem, any compounds or agents that can help improve the survival of implanted cells in this condition can be used for nerve regeneration (16). In this regard, phenytoin (PHT, 5,5-diphenylhydantoin, DPH) is one of the main antiepileptic drugs. The antiseizure action of phenytoin is probably due to the obstruction of voltage-gated sodium channels (17, 18). A large number of investigations have shown that phenytoin inhibits many presynaptic and postsynaptic Ca2+-mediated phenomena (19-21). Also, phenytoin decreases neurotransmitter release, reduces calcium influx, and decreases excessive spiking in nerve cells (22). Some studies have described the cerebroprotective effects of phenytoin in an established model of global cerebral ischemia (19, 23).
2. Objectives
In this study, the supportive effects of phenytoin were evaluated on the cultivation and maintenance of stem cells (WJSCs) on sciatic nerve acellularized scaffolds.
3. Methods
3.1. Animals
In this experimental study, 15 male Wistar rats with an approximate age of two months and a weight of 200 - 250 g were used as sciatic nerve donors. Animals were sacrificed, and their sciatic nerves were harvested for the preparation of acellular nerve allograft (ANA) under sterile conditions. Prepared acellular nerve allografts were randomly divided into three groups (n = 20). In the 0.5 µg/mL experimental group, cultured cells on ANA were treated with 0.5 µg/mL phenytoin for eight days. In the second experimental group, cultured cells on ANA were treated with 1 µg/mL phenytoin for eight days. In the control group, cultured cells on ANA did not receive any medication.The selected doses of phenytoin showed anticonvulsant and cerebroprotective effects in previous studies (19, 22). All animal experiments were carried out following the European Communities Council directive of 24 November 1986 (86/609/EEC) and in accordance with the local committee of the University of Mohaghegh Ardabili (UMA) for Human and Animal ethics.
3.2. WJSCs Preparation, Characterization, and Culture
In this experimental study, umbilical cords required for stem cell extraction were obtained from the Obstetrics Department, Ghaem Hospital, Ardabil University of Medical Sciences. To extract human umbilical cord WJSCs, the umbilical cord was first sterilized in 70% ethanol for 30 seconds. Then, 20 mm fragments of the cord were placed in HBSS, and the umbilical cord vessels were removed from the Wharton’s jelly tissue. In the next step, the mesenchymal tissue was cut into 0.5 mm pieces. The pieces were then cultured on a 75 cm2 flask (SPL, Korea) containing culture media (DMEM) with 20% FBS and 1% penicillin and streptomycin (24, 25). The flasks were incubated in a standard incubator under a standard condition of 5% CO2 at 37°C, and the cell media were replaced every three days. Passage 3 WJSCs were cultured for 21 days in the osteogenic differentiation medium and the presence of calcium deposits in the extracellular matrix, cells cultured using Alizarin Red staining was investigated after 21 days. Furthermore, WJSCs at passage 3 were cultivated for 21 days in the adipogenesis differentiation medium and differentiated WJSCs were stained by Oil Red O dye. To verify the WJSCs phenotype surface marker of cells, cells in passage 3 were evaluated by flow cytometry (as described by the manufacturer) for the expression of cell surface markers such as CD73, CD44, CD90, CD105, CD34, and CD45. The cells were first separated from the flask by trypsin. Then, the cell suspension was counted and transferred to plates containing 1 mL of 1% PBS-BSA solution for 30 min. Cell suspensions were centrifuged, and appropriate concentrations of isotype control, CD34, CD44, CD45, CD73, CD90, and CD105, antibodies were added according to the procedure. Next, the cells were washed and centrifuged in a dark place at 4°C with PBS. The resultant cell suspension was suppressed with 200 μL of FACS buffer and stored at a temperature of 2 - 8°C during the examination and read with a flow cytometer. Flow cytometry was done using a flow cytometer (BD FACSCalibur), and the results were analyzed by Flow Jo software version 10.
3.3. Preparation of ANA
The Sondell method was used to prepare the acellular nerve allograft from the sciatic nerve of rats. In the first stage, the sciatic nerve pieces were immersed for seven hours in distilled water. Then, the segments were placed for 12 hours in 3% Triton X-100. Subsequently, the segments were subjected to 4% sodium deoxycholate (Sigma) for 24 hours. The three mentioned steps were repeated twice at room temperature. Finally, acellular nerve allografts were washed with distilled water and stored in PBS before use (26, 27).
3.4. Tensile Testing of ANA
The intact nerve and acellular nerve allograft samples underwent biomechanical examinations. Samples with an average length of 1 cm that were constantly moistened with PBS were subjected to a pressure of 0.05 mm/s by a Universal Testing Machine (SANTAM-STM20, Tehran, Iran) and this traction continued until the samples were torn (28).
3.5. Histological Evaluation of ANA
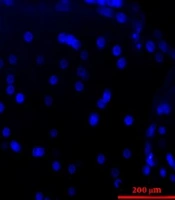
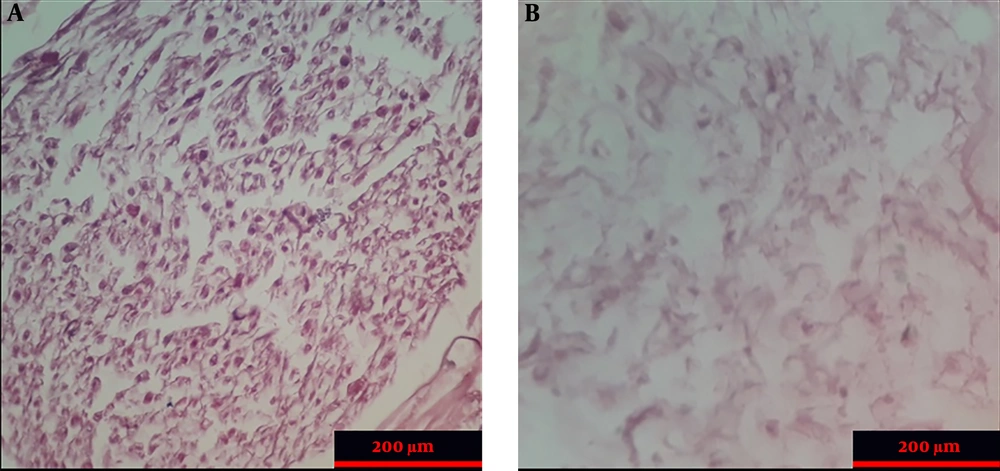
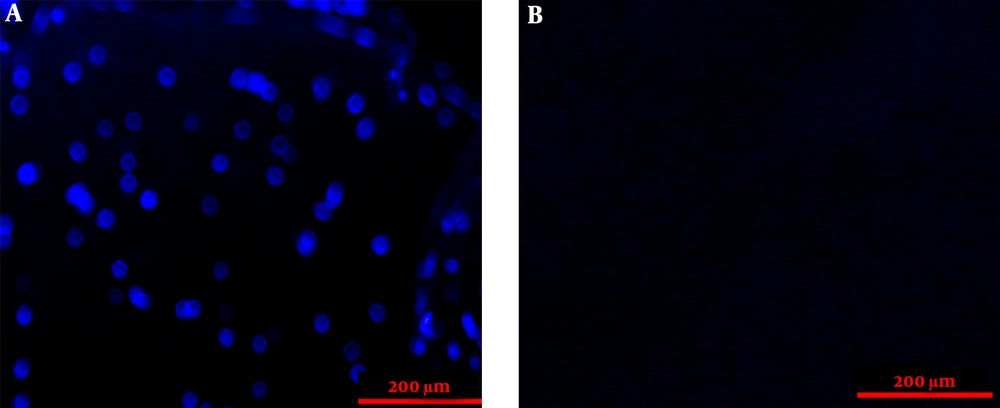
Hematoxylin and eosin (H&E) staining was used to examine the structure and characteristics of the tissue. Thus, acellular nerve allograft and intact nerve histological segments were stained using H&E to observe customary morphology. First, the samples were fixed in 10% formalin. The samples from the natural nerve and the acellular scaffold were placed in the xylene solution and then in different degrees of ethanol. Xylene caused deparaffinization, and different concentrations of ethanol caused the samples to be rehydrated. Finally, slides stained with H&E were examined using an optical microscope (Olympus, Japan). The presence and strength of collagen fibers in intact nerve and acellular nerve allograft samples were compared using van Gieson's picro-fuchsin staining (29). Also, to investigate DNA fragments, the slides prepared from intact nerve and acellular nerve allograft samples were stained with DAPI and after 15 min, evaluated by a fluorescent microscope (Olympus, Japan).
3.6. Effects of Phenytoin on Viability and Proliferation of WJSCs in Normal Condition
The MTT assay was used that is based on the mitochondrial activity of cells so that the higher activity of cell mitochondria represents the greater proliferation of cells. In addition, this method is used to measure the cytotoxic effects of drugs on different types of cells. In this study, 1 × 106 cells within microplates were exposed to certain doses of phenytoin for 24 hours. The cell culture medium was discarded after four hours of incubation with 20 μL MTT reagent (5 mg/mL), and then DMSO (200 μL) was added and remained for one hour. The MTT gives a yellow solution which, with activity of dehydrogenases enzyme in metabolically active WJSCs, produces a water insolvable violet-blue formazan.Then, the absorbance was read by an ELISA reaeder for each sample at a 570 nm wavelength.
3.7. Role of Phenytoin in Proliferation and Retention of WJSCs on Scaffold
To investigate the effects of phenytoin on maintaining morphology and cellular adherence of WJSCs on the acellular nerve allograft, first, 1 × 106 WJSCs were seeded onto each of the provided acellular nerve allograft using a stereomicroscope (Nikon, Japan). Scaffolds containing cells were allowed to attach at 37°C for 4 hours and then incubated in a standard incubator under a standard condition of 5% CO2 at 37°C for eight days (30). Afterward, the growth and proliferation of cells on the acellular nerve allograft was measured in the presence of phenytoin on the eighth day using the MTT assay. In addition, on the eighth day, to evaluate the cell interactions with scaffolds, scaffolds with seeded cells were fixed bya glutaraldehyde (4%) fixator at 4°C for 12 hours. The cellular connections to scaffolds were observed using an SEM device.
3.8. Statistical Analysis
The data from this experimental study were analysed by SPSS (version 19.0). The comparison of data between the groups were done using the one-way Analysis of Variance (ANOVA), Also, the Tukey test was done for analysing the differences. The p value of < 0.05 was considered significant.
4. Results
4.1. Tensile Testing of ANA
The tensile testing result showed that there was no significant difference in the length and width of samples between ANA and control group. Also, the biomechanical properties of the studied groups were not decreased (Table 1). The tensile testing results demonstrated a significant difference in the average breaking stress between ANA and intact nerves (P < 0.05) (Table 1).
Comparison of Biomechanical Properties of Fresh Nerve and Acellular Nerve Scaffold (n = 5).
4.2. WJSCs Characterization
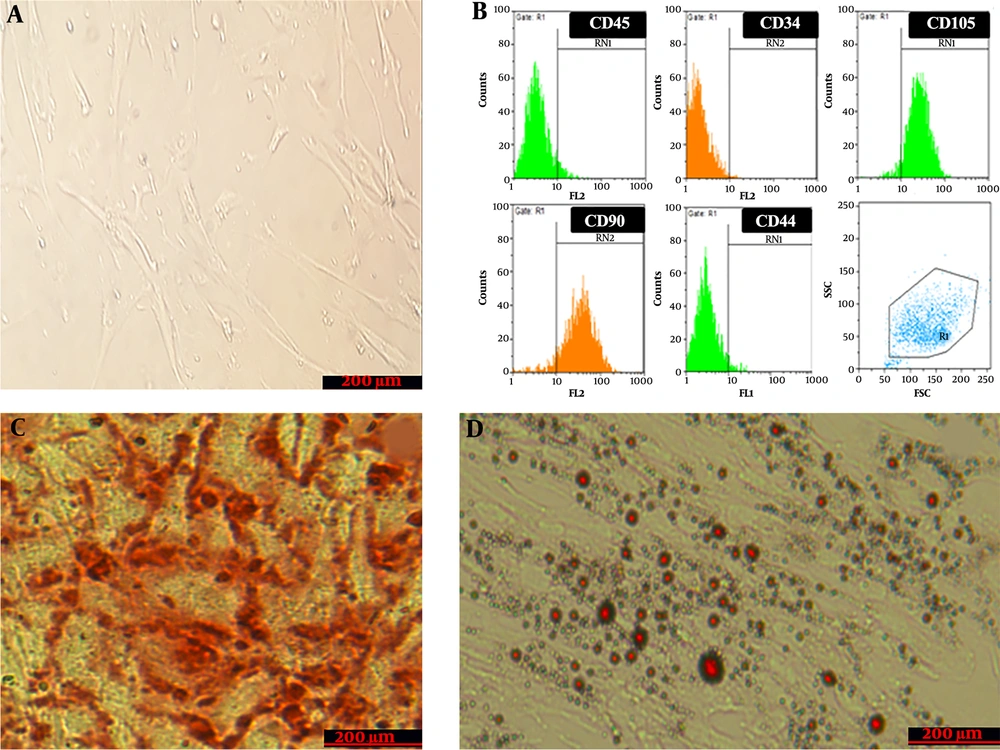
WJSCs at passage 3 were observed by inverted-phase microscopy. The results showed that cells attached to the floor of the flask and had a fibroblast-like morphology (Figure 1A). The examination of WJSCs at passage 3 by flow cytometry showed that expression was negative for CD34 (0.67 ± 0.02%) and CD45 (5.57 ± 0.2%) as hematopoietic markers and positive for CD73 (95.5 ± 1.3%), CD44 (94.5 %), CD105 (93.92 ± 0.81%), and CD90 (89. 3 ± 0.67%), which are an evidence of the mesenchymal properties of these cells (Figure 1B). Also, the differentiating ability of WJSCs was examined in an osteogenic differentiation and adipogenic differentiation medium by special staining for the mineralization of calcium (Figure 1C) and intracellular lipid vacuole (Figure 1D), which confirmed the multipotent potential of WJSCs.
Characteristics of wharton’s jelly stem cells; A, Morphology of cultured cells. Cultured cells under invert microscopy have a spindle shape; B, Flow cytometry analysis of wharton’s jelly stem cells surface-marker expressions; C, Alizarin red staining for evaluating the osteogenic differentiation capacity of stem cells. The figure shows calcium crystal formation that confirms the differentiation; D, Oil-red-O staining for evaluating the Adipogenic differentiation capacity. The figure shows lipid droplet formation that confirms the differentiation.
4.3. Characterization of ANA
The histopathologic examination of control and ANA samples showed that ANA samples were white and transparent when compared to control samples. In addition, the flexibility and length of ANA were slightly lower than those of control samples. The results of H&E staning of specimens demonstrated that nerve cells were seen in the extracellular matrix of the intact nerve, while ANA samples lacked these cells (Figure 2). The results of the Van Gieson test confirmed that the extracellular matrix structures (collagen and elastin fibers) were largely preserved in ANA, similar to intact nerves. The results of the DAPI test demonstrated that the DNA observed in intact nerve samples was not seen in ANA samples (Figure 3).
4.4. Tensile Testing of ANA
The tensile testing result showed that there was no significant difference in the length and width of samples between ANA and the control group. Also, the biomechanical properties of the studied groups were not decreased (Table 1). The tensile testing results demonstrated a significant difference in average breaking stress between the ANA and intact nerve (P < 0.05) (Table 1).
4.5. Effects of Phenytoin on WJSCs Viability and Proliferation
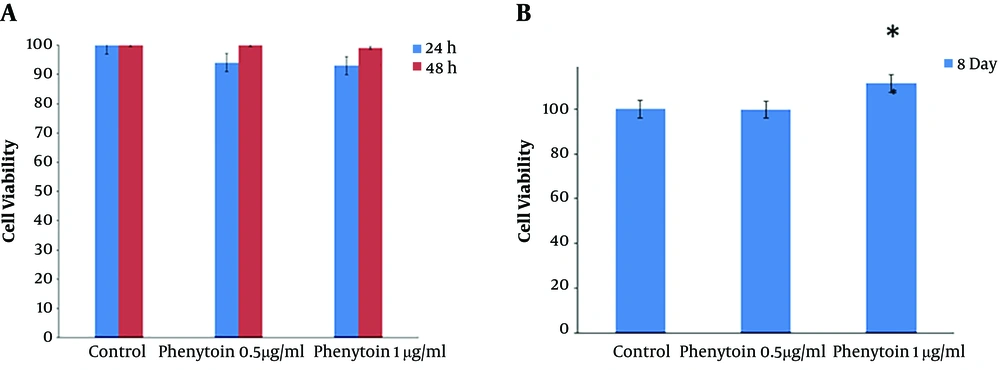
The WJSCs viability and proliferation rate were examined in three groups, including the control group and two groups with different concentrations of phenytoin (0.5 μg/ mL and 1 μg/ mL) using the MTT assay after 24 and 48 hours. The results showed that the different doses of phenytoin did not have toxic effects on cultured WJSCs (Figure 4A).
A, Effects of phenytoin on WJSCs. The results of cultured cells in the MTT assay demonstrate that phenytoin under standard culture conditions has no toxicity on cell viability. B, Effects of phenytoin on viabilty and retention of cultured cells on the scaffolds showed that after eight days in the phenytoin-treated group (1 µg/mL), cell viability, proliferation, and adhesion increased significantly when compared to other groups (P < 0.05).
4.6. Effects of Phenytoin on the Viability and Retention of WJSCs Seeded on Scaffolds
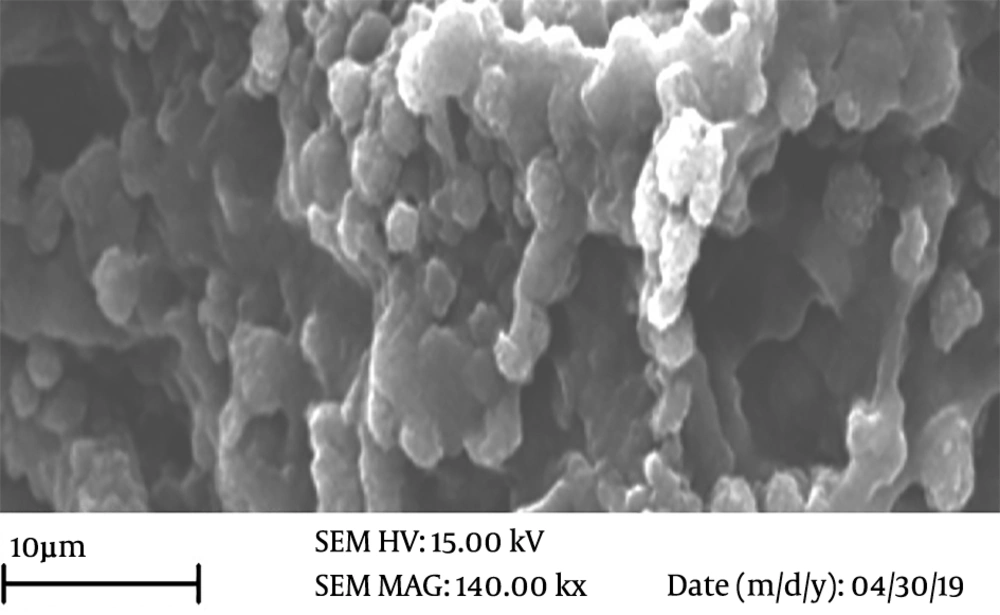
To check the attachments and persistence of WJSCs on the ANA, the MTT assay and SEM images were used eight days after the cells were seeded on scaffolds. The MTT assay results showed that the ANA did not have any toxic effects on WJSCs. Also, there was a meaningful increase in the viability and proliferation of cells in the 1 μg/mL phenytoin group when compared to other experimental groups (P < 0.05) (Figure 4B). We used SEM to appraise the adhesion and persistence of WJSCs on the scaffold. The SEM images demonstrated that cultured cells well sticked to the prepared scaffolds (Figure 5). Additionally, the SEM images demonstrated that cultured cells on the scaffolds were distributed uniformly throughout the ANA membrane.
5. Discussion
The rapid regeneration of peripheral nervous system injuries is one of the important challenges in neurobiology and neurosurgery 30. In this study, we used the Sondell method for decellularization of nerves. The results of histological tests (H&E and DAPI) demonstrated that following the decellularization process, there were no cells and DNA components in the prepared scaffolds, and they were eliminated completely, while the natural structure of ECM was well preserved in the prepared scaffolds. The results of the tensile test showed that the ECM components did not change remarkably after decellularization. The MTT results demonstrated that the viability and proliferation of cells were higher in the 1 μg/mL phenytoin group than in the other groups. Also, SEM micrographs in this study showed that the ANA prepared by the Sondell method provided an appropriate microenvironment for cell adhesion and phenytoin increased cell viability, cell proliferation, and retention by regulating the ionic balances in the scaffold.
In tissue engineering, one of the key goals is to produce a three-dimensional scaffold that imitates the extracellular matrix and allows for cell growth, proliferation, differentiation, and migration (31). The acellular matrix produced by tissue engineering can provide attractive and 3D scaffold conditions for successful cell binding (27, 32). In a study conducted by Ghayour et al., a new treatment with the combination of ANA and adipose stem cells showed to be a suitable alternative for nerve autografts and could be used for the regeneration of peripheral nerve injureies in a rat model (28). Additionally, acellular nerve grafts have an internal structure essentially tantamount to that of normal nerve tissue. The main purpose of producing these grafts is to reduce the incidence of immunogenic response, which is done through cell removal (12). Although the perfect removal of cellular components for the preparation of acellular scaffolds in tissue engineering is essential, the maintenance of ECM is important because its existence is essential for the secretion of growth factors and provide the microenvironments for tissue regeneration, growth, proliferation, and cell adhesion. Previous reports demonstrated that the ECM has a very important role in the regeneration of peripheral nerves and can provide the Bungner band for Schwann cells (33). Moreover, some evidence suggests that extracellular matrix complex elements, such as heparan sulphate, laminin, proteoglycans, some types of collagen, and fibronectin, can improve early recovery stages following nerve injuries (27, 33). Concerning biomechanical tests, previous studies have shown that retaining the ECM components after decellularization is very important to preserve the mechanical properties of scaffolds and that damage to scaffolds during the decellularization process decreases the mechanical strength of scaffolds (34, 35).
Consistent with our findings, previous studies demonstrated that calcium has an irreversible role in cell death (36, 37). Therefore, phenytoin, by eliminating calcium from the extracellular environment, reduces cell death. Other studies reported that axonal injury could induce the influx of sodium ions through sodium channels. This influx of sodium ions can open other channels such as calcium channels and increase intracellular calcium. This could result in ionic imbalances in the injury site (38, 39). Also, another study showed that treatment with phenytoin after ischemia in rabbits afforded a significant protection of neurons in the hippocampus (40).
5.1. Conclusions
The study showed that the ANA prepared by the Sondell method provides an appropriate microenvironment for cell adhesion, and phenytoin at a concentration of 1 μg/mL increases the viability, retention, and proliferation of cells. Thus, these results suggest that phenytoin can be considered A new treatment for nerve regeneration and tissue engineering applications.