1. Background
Esophageal cancer is an aggressive malignancy with high mortality. Despite improvements in treatment approaches, its prognosis and survival rate remains low (1, 2). The treatment approaches include esophagectomy, chemotherapy, and radiotherapy. However, about 85% of esophageal cancer patients die two years after diagnosis (3). Hence, it is crucial to introduce new, low-invasive, and more useful therapeutic methods (4, 5).
Studies show that the consumption of fruit and vegetables could decrease the risk of cancer development. Several studies have recently focused on plant-foods containing polyphenolic compounds, which generally target multiple signaling pathways to inhibit cancer progression (6-8). Studies in the past decade show the useful properties of pomegranate components (9-11). The peel contains 50% of the total weight of pomegranate. It is the source of valuable compounds (e.g., phenols, flavonoids, proanthocyanidins), minerals (e.g., potassium, nitrogen, calcium, phosphorus, magnesium, and sodium), and also complex polysaccharides. The fraction of pomegranate (50%) also contains the edible seeds (40%) and the seed kernel (10%). The kernel of seeds is a rich source of total lipids. The pomegranate oil contains 12 - 20% of the total seed weight (10, 12-15). Pomegranate seed oil includes a high content of unsaturated fatty acids (e.g., linolenic, oleic acid, starchy acid, palmitic acid, punicic acid), which targets breast cancer cells and kills them usefully (11, 16). It also contains antioxidant, anti-carcinogenic, antibacterial, and anti-inflammatory components, which can prevent and treat several cancers, such as colon, lung, and prostate cancer (11, 17-21).
2. Objectives
The molecular mechanism of different cancers is different, so their response to natural treatment (such as pomegranate oil) could be different. Therefore, it is significant to assess the effect of the pomegranate component on esophageal cancer. Given the significant features of pomegranate and the high prevalence of esophageal cancer in Iran, the present study aimed to evaluate the anti-cancer properties and cytotoxic effects of pomegranate seed oil against the esophageal cancer cell line.
3. Methods
3.1. Preparation of Pomegranate Seed Oil
After separating the seeds of pomegranate and washing them with distilled water, the seeds were exposed to the sun to dry and then powdered into a grinder. Then, for every 60 g of powder, 300 ml of petroleum ether (Sigma-Aldrich, Germany) was added and shacked for 24 h. Subsequently, the mixture was sprayed with a flat paper filter, and the obtained solution was incubated for 24 hours at 37°C. On average, each 30 g of pomegranate powder produced 2 g of oil. Then, 2,000 mg of oil was dissolved in 1 mL de methyl sulfoxide (DMSO) (Merck, Germany). Pomegranate powder was used at the final concentration of 0.1% in the culture medium to avoid the toxicity effects of DMSO (22-24). Finally, the solution was sterilized with a 0.2 nm filter (25-28).
3.2. Cell Culture
The human esophageal cancer cell line of KYSE-30 and the HF2FF fibroblast cell line, as normal cells (25, 29, 30), were purchased from the Pasteur Institute of Iran. The KYSE-30 cells were cultured in RPMI-1640/Ham's F12 (Gibco, New York, USA), and the HF2FF cells were seeded in RPMI-1640 (Gibco, New York, USA) medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Bio Sera, Kansas City, MO, USA) (31-34), 100 U/mL penicillin, and 100 mg/mL streptomycin (Gibco, New York, USA). The cultured cells were stored at 37ºC and 5% CO2 in the incubator. The culture medium was replaced 2 - 3 times a week. The cells were passaged twice to adapt to the culture condition.
3.3. Analysis of Pomegranate Oil Toxicity Using MTT Test
We used the MTT colorimetric test using tetrazolium, 3- (4,5-dimethylthiazol-2yl) -2,5-diphenyl tetrazolium bromide (Sigma-Aldrich, St. Louis, MO, USA) to investigate the cytotoxicity effects of pomegranate oil on normal and cancer cells. After two passages, the cultured cells of KYSE-30 and the HF2FF were collected and counted. Then, a total of 104 cells per well were cultured in 96-well plates and treated at 75% confluence with different concentrations of pomegranate oil (2000, 1000, 500, 250, 125, 62.5, 31.25 μg/mL) for three periods of time (24, 48, 72 h). For each concentration, three repeats were considered. After the proper time of incubation, the supernatant was removed, and the cells were washed with PBS. Then, 50 μL of MTT solution (5 mg/mL) was added to each well and stored for 3 h at 37°C. After incubation, 100 μL isopropanolic acid (Merck, Germany) was added to each well and slowly pipetted to resolved formazan crystals. The intensity of the color was then measured by the ELISA reader (Biotek, Germany) at 570 nm. The results were calculated based on the percentage of cell viability. For each concentration, we used a blank (a well without cultured cells) and negative control (a well with cultured cells and without pomegranate oil). All tests were performed in triplicate and were repeated three times. The cellular viability was estimated as % compared to untreated cells.
3.4. Wound-healing Assay
A wound-healing assay was used to determine the mobility and migration potential of the cells (35, 36). Briefly, 106 KYSE-30 cells were cultured in 24-well plates for 24 h to create a confluent monolayer cell culture. Then, the wounds were created by scraping across the cell monolayer using a micropipette tip. Monolayer cell culture medium was immediately replaced with fresh medium, and the wounded cells were treated with three concentrations of pomegranate seed oil or DMSO alone as a control sample. Finally, the amount of cell migration for wound closure was monitored for 24 h. Phase-contrast images were obtained at the time of wounding (0 h) and after 24 h.
3.5. Statistical Analysis
All tests were repeated three times, and the results were analyzed using SPSS 22 software. All data were expressed as Mean ± SD. Also, the IC50 (half maximal inhibitory concentration) value was calculated via plotting the graph of concentration against the growth inhibition (x-y graph) using the value 50 for Y in the linear equation of the graph (Y = mX + n). Statistical analysis was performed by one-way ANOVA test, and P-value less than 0.05 was considered statistically significant.
4. Results
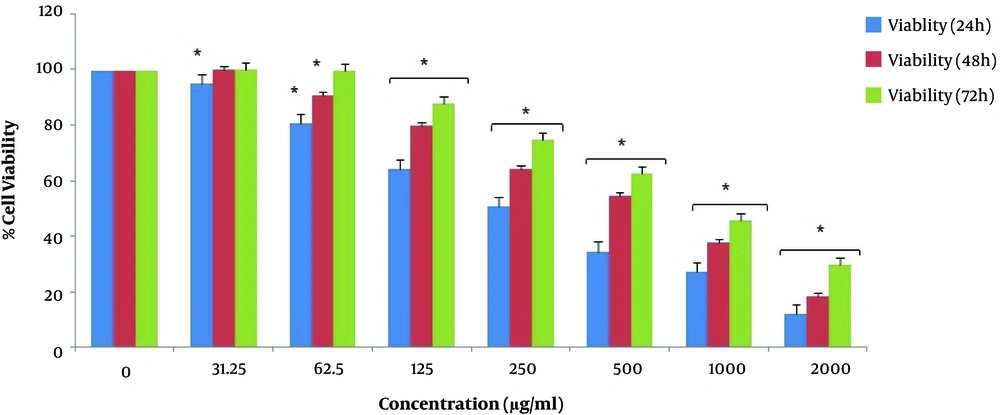
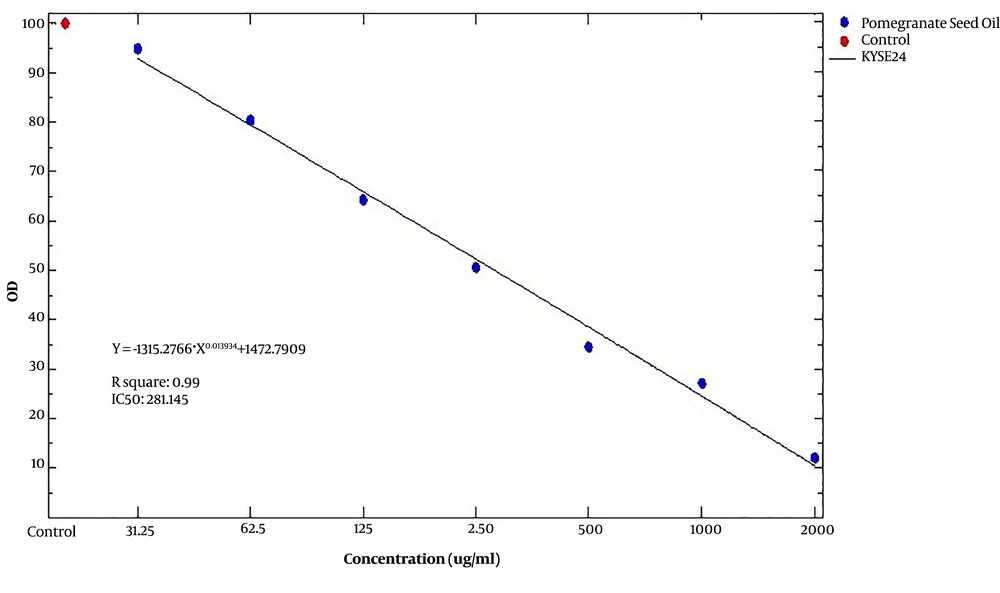
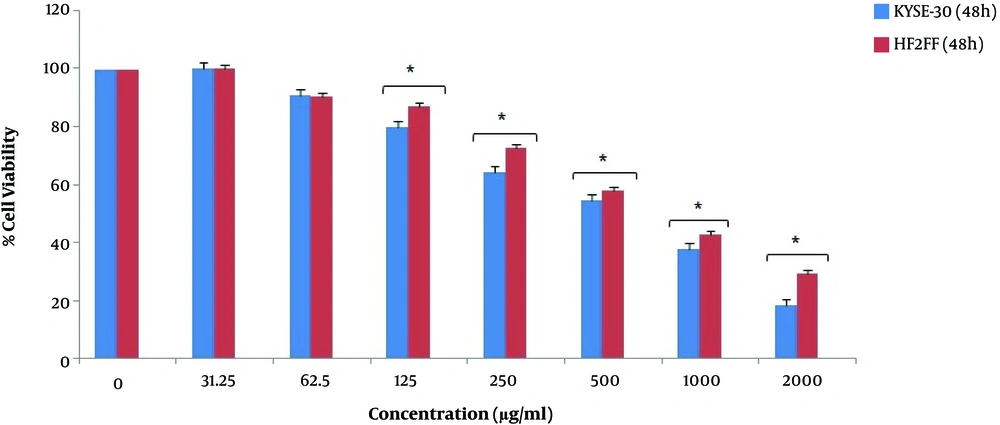
We investigated the viability percentage of KYSE-30 cells after treatment with seven different concentrations of pomegranate oil for 24, 48, 72 h, and obtained significant results. According to Figure 1, the growth rate of KYSE-30 cells treated with different concentrations of pomegranate oil was significantly decreased compared with the control group (P < 0.05). It seems that the concentration of 2000 μg/mL had the most cytotoxicity effect on cancer cells. Also, the viability rate of esophageal cancer cells depends on the dose of pomegranate oil as well as the duration of treatment as well. The cancer cell viability was decreased significantly after 24 h of treatment. However, the percentage of cell death was decreased along with increasing the treatment time. The most cytotoxicity has been observed after 24 h, and lower cell death occurred after 48 h and 72 h of treatment (Figure 1). The IC50 value of pomegranate seed oil was 281.14 μg/mL in 24 h (Figure 2), 549.72 μg/mL in 48 h, and 850.18 in 72 h of treatment (Table 1).
| Time of Treatment | IC50 (μg/mL) |
|---|---|
| 24 h | 281.14 |
| 48 h | 549.72 |
| 72 h | 850.18 |
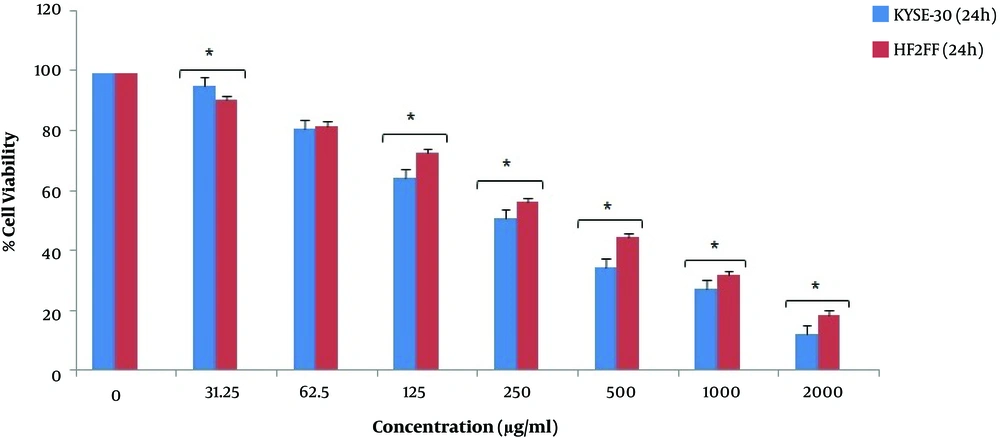
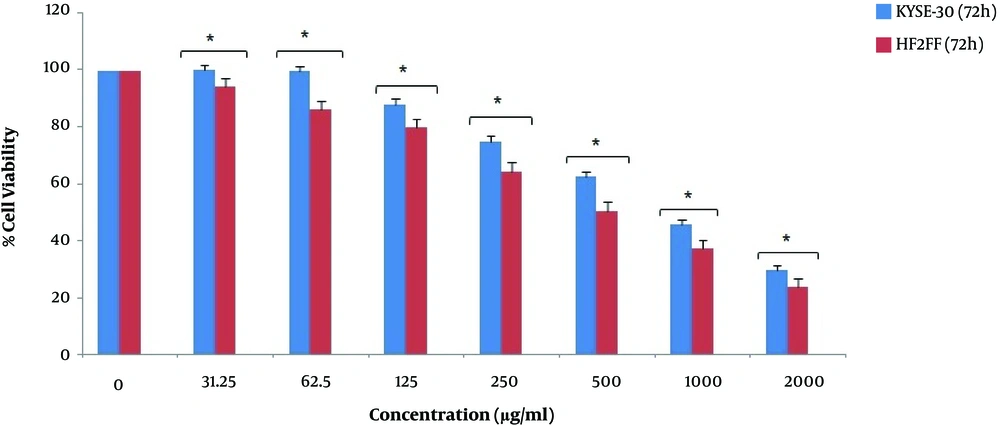
According to the results, the viability of HF2FF cells decreased after different concentrations of pomegranate seed oil. However, the decrease rate of HF2FF cells was significantly lower than KYSE-30 cancer cells at 24 h and 48 h (P < 0.05). As shown in Figure 3 and Figure 4, the viability decrease rate of HF2FF cells was less than the KYSE-30 viability decrease rate after 24 and 48 h (Figure 3 and Figure 4). However, according to Figure 4, the survival and viability rate of HF2FF cells was less than the KYSE-30 cells after 72 h of treatment (Figure 5).
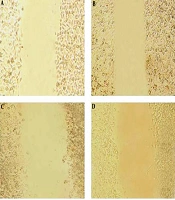
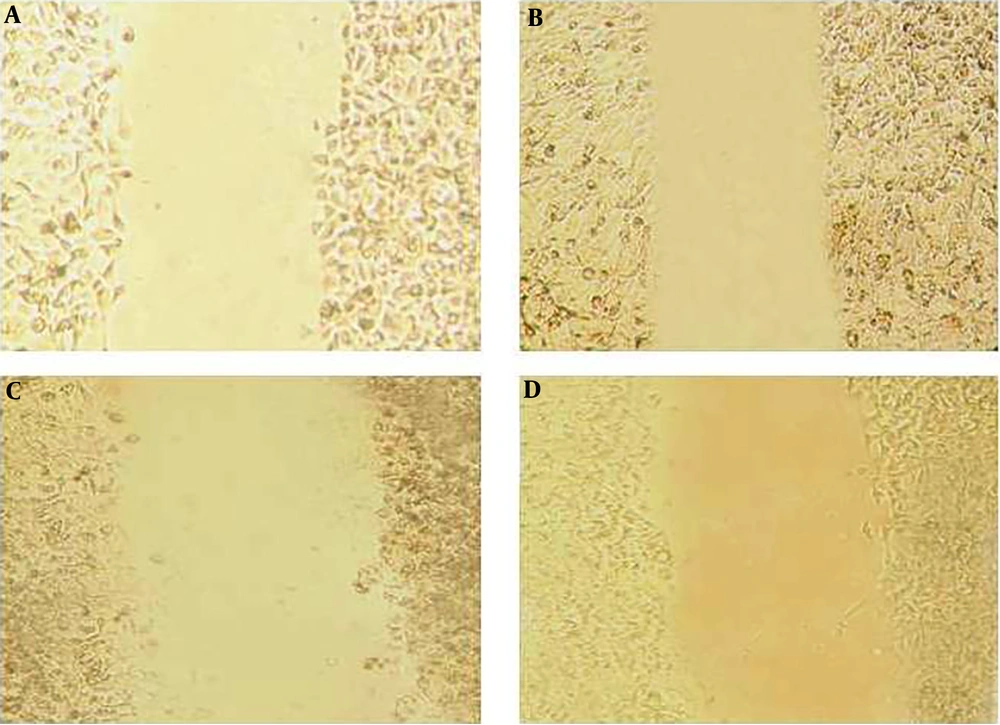
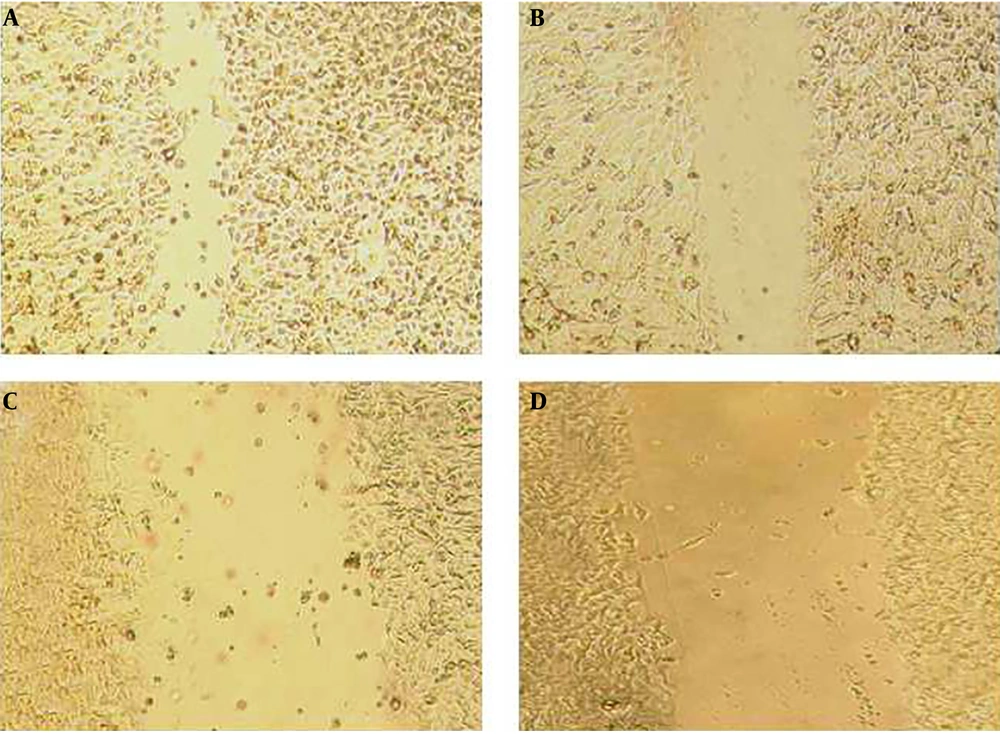
The wound-healing assay was used to evaluate the effect of the pomegranate oil on cancer cell migration. The assay was done by making a scratch on a cell monolayer, which produces a cell-free area, and then capturing microscopic images at regular intervals. Thus, the cells with migration ability could migrate and close the gap. The results of the wound-healing assay indicated a decrease in the mobility and migration of esophageal tumor cells after treatment with different concentrations of pomegranate seed oil. As shown in Figure 6 and Figure 7, the untreated control cells had migrated into the wounded cell-free area of the cell monolayer. However, the migration ability of treated tumor cells was decreased along with increasing the oil concentration, in which the wounded area remained empty in the group treated with 2000 μg/mL (Figure 6 and Figure 7).
Effect of pomegranate seed oil on KYSE-30 cell migration. Pomegranate seed oil could reduce cell migration and wound closure in KYSE-30 cells relative to control cells. Images of scraped KYSE-30 cells treated with different concentrations of pomegranate seed oil after 24 h. A: Control sample, B: Concentration 500 μg/mL, C: concentration 1000 μg/mL, D: concentration 2000 μg/mL.
5. Discussion
Cancer, as one of the fundamental causes of death, is increasing worldwide. According to the International Agency for Research on Cancer (IARC), esophageal cancer is the world’s eighth most common and deadly cancers occurring at the highest incidence rate in countries located in the Asian esophageal cancer belt (2). Golestan province in Iran has been long known as one of the areas with a high incidence of gastrointestinal cancers (37, 38). Esophageal cancer is now the most common cancer in Golestan province, and its occurrence is reported more than in other parts of the world (38). Various studies have attempted to identify new diagnoses and treatment approaches for esophageal cancer (4, 39).
It is quite challenging to treat esophageal cancer with current therapies. Thus, applying natural-originated factors such as plant compounds can be considered as a new strategy. According to numerous studies on pomegranate juice, pomegranate seed oil can have anti-cancer properties. We examined the effect of pomegranate seed oil on KYSE-30 cells due to the high prevalence of esophageal cancer in Iran.
The results of this study indicate that the high potency of pomegranate oil can inhibit the growth of KYSE-30 tumor cells in comparison with the control cells, which is dependent on the dose and time. The most cytotoxicity of pomegranate oil occurred after 24 h, and lower cell death occurred after 48 h and 72 h of treatment. Similarly, several studies have reported a lower cytotoxicity level in more prolonged treatments (40-42). Furthermore, Bajbouj et al. showed that the viability of colon cancer cell line treated with saffron extract was less at 24 h than 48 h due to the early upregulation of cycline B1 at 24 h of treatment and its subsequent downregulation was at 48 h (43).
Therefore, the viability decrease observed in tumor cells indicates the potential of this compound as a new and complementary therapeutic strategy for esophageal cancer.
The wound-healing assay can evaluate the effect of pomegranate oil on cellular mobility and cell migration. It confirmed the role of this substance in inhibiting the mobility and migration of esophageal tumor cells. Therefore, it seems that pomegranate seed oil can function as an inhibitor of metastasis and invasion of esophageal tumor cells.
Studies have shown that biochemical compounds of pomegranate fruit contain an immense number of anthocyanins, which have potent antioxidant activity. Pomegranate juice and pomegranate oil have antioxidant and anti-inflammatory properties (9, 11, 16, 44). Clinical studies have shown that pomegranate can prevent cancer cell growth in the skin, breast, and prostate (18, 45, 46). Several in vitro studies have also shown the therapeutic effects of pomegranate extracts on various cancer cells such as breast cancer (MB-MDA and MCF-7), oral cancer (CAL-27 and KB), colon cancer (HT-29), and lung cancer (19-21, 47).
Moreover, the effects of pomegranate extract on prostate cancer indicate its high potential in inhibiting cancer metastasis and its significant cytotoxicity effect on the cancer cells (48). These observations suggest that pomegranate and its derivatives can act as a potential source for cancer treatment (49).
Gomes et al. found that pomegranate juice extracts had anti-proliferative and pro-apoptotic effects in human pancreatic cancer cells (50). Also, this extract can increase the binding and adhesion of cancer cells by regulating the adhesion proteins, which in turn leads to reducing the mobility and invasion of cancer cells (50). The pomegranate derivatives have anti carcinogenesis effects on the digestive tract cancer cell lines, including gastric adenocarcinoma and liver tumor cell line (16).
Another study focused on the effect of the Punicic acid toxicity of pomegranate oil, revealing a dose-dependent cytotoxic effect of the Punicic acid on the leukemia cell line (K562) (51). The polyphenolic content of pomegranate and the unique combination of its fatty acids can have anti-inflammatory, antiviral, antibacterial, and anti-fungal properties (16, 18, 21, 46). Moreover, Hora et al. have shown the chemopreventive efficacy of Pomegranate seed oil in skin cancer (52).
5.1. Conclusion
Pomegranate seed oil can have significant effects on inhibiting the viability as well as reducing the migration ability of esophageal cancer cells. Therefore, due to the nature of the product, low cost, and availability, the use of this dietary supplement may be useful for esophageal cancer treatment. However, applying the pomegranate seed oil as a new or complementary treatment option for esophageal cancer requires more comprehensive clinical studies.