1. Background
The propagation of drug- and antibiotic-resistant microbial isolates is a serious concern in the food and pharmaceutical industries. Today, medicinal plants, which are used to treat chronic diseases, have drawn the interest of many researchers because of the toxic and carcinogenic properties of their chemical and biological compounds. A good and healthy substitute for foods may be natural antimicrobial and antioxidant compounds such as organic acids, fragrances (essential oils), and plant extracts (1). The phenolic compounds and antioxidants of these plants (known as secondary metabolites) have been shown to prevent oral diseases and arthritis, where collagen is damaged (2).
Plant secondary metabolites have been used to cure infections and diseases since the beginning of human life. The synthetic chemical drugs (such as aspirin and salicylic acid) designed based on plants’ chemical compounds have now substituted natural products (3-5). Considering plants as factories for producing various biochemical products, new research efforts have been made to develop secondary metabolites as sustainable natural products upon the increasing demand of the market in recent years (3, 6). Since the second half of the last century, extensive research has been conducted on medicinal plants in most countries, resulting in the production and marketing of many herbal medicines. Iran’s plant flora, which includes more than 7500 plant species, provides a rich source for many medicinal herbs. So, it is essential to study the biological properties of the effective substances of Iran’s natural plants (7, 8).
Some major clinical diseases can be prevented by using natural citrus and vegetable-derived antioxidants. Some studies have verified the association between fruit and vegetable intake and reduced risk of chronic diseases (9). Nevertheless, fruits and vegetables are diverse in terms of their antioxidant compounds and antioxidant activities (10, 11).
Escherichia coli (Gram-negative Bacilli) is one of the most serious pathogens in poultry, inflicting huge economic losses on the poultry industry. The indiscriminate use of antibiotics has resulted in today’s growing prevalence of drug-resistant bacterial strains. In fact, the spread of these strains tends to occur much faster than the discovery of new drugs. Therefore, several attempts have been made to find new compounds as effective alternatives to antibiotics. Medicinal plants constitute a rich and valuable source for various compounds with antimicrobial properties and can be regarded as viable choices to replace antibiotics. There has been a surge in the emergence of antibiotic-resistant E. coli in poultry. On the other hand, Eshvarak, a plant growing in Iran, contains various enzymatic and non-enzymatic antioxidants and has traditionally been used to treat a variety of diseases. So, this study aimed to evaluate the antimicrobial effects of the ethanolic, methanolic, and ethyl-acetate extracts of this plant against the E. coli isolated from quail feces.
Eshvarak is a genus of oleanders (Rhazya stricta Decne), a 50 -100 cm tall shrub that grows in hot and dry regions. In Iran, its growth is confined to the country’s southern areas. Her leaves are long, pointed, leathery, and thick, and flowers are white or yellow and appear either next to the stem or at the end of branches. In the Middle East, Eshvarak is distributed especially in the southern and southeastern regions of Iran. The habitats of this plant are Sistan and Baluchestan, and Hormozgan provinces of Iran. It also grows in Afghanistan, Pakistan, and Saudi Arabia (12). For insects, Eshvarak has lethal, repellent, and anti-nutritional compounds (13). The plant has been used for treating sore throat, acne, toothache, fever, mouth blisters, sunburn and electrocution, rheumatism, thirst, and acne, and small blisters of the mouth and gums, as well as for the germination of children’s teeth. Rubbing the juice of fresh Eshvarak leaves and infusing its leaves have been shown to eliminate pimples on the body’s skin. Eshvarak sap is used as a remedy for toothache and to induce the timely growth of children’s teeth. Furthermore, its calyx has been used to quench thirst in the desert (12). To date, Eshvarak extracts, fragrances, and alkaloids have been used to cover wounds on the palm tree’s trunk to avoid spawning of fawn heads and reduce new pest infections (13). These compounds have been used as potent antimicrobial and antifungal agents (12, 14-17) and to synthesize silver nanoparticles (18).
2. Objectives
Due to the growing antibiotic resistance of E. coli in poultry, this research was conducted to determine the antibiotic resistance pattern of the E. coli isolated from quail fecal samples and to investigate the antimicrobial effects of Eshvarak extracts against this bacterium.
3. Methods
3.1. Preparation of Plant Extracts
Eshvarak plant was collected from Saravan (coordinates: 27°22′15″N; 62°20′03″E, Sistan and Baluchestan province) and identified in the botany laboratory of the University of Zabol. Ten g of the plant’s leaf (Figure 1) was placed in the shade and dry air. After milling, it was extracted using 100 mL of various solvents (methanol 100%, ethanol 100%, distilled water, hydro-alcohol (70 % aqueous and 30 % ethanol), and ethyl-acetate 100%). The plant was soaked at room temperature for 48 hours and deposited in a shaker. Afterwards, extracts were refined, and the solvent was allowed to evaporate at < 40°C in a rotary evaporator. After drying, the residue was stored in a refrigerator at 4°C for future experiments (19).
3.2. Bacterial Isolates
Escherichia coli strains were isolated from quail fecal samples in Zabol city and cultivated in nutrient agar. The isolated bacteria were identified by a variety of biochemical, bacteriological, and growth tests (oxidase, catalase, glucose tests such as lactose, sucrose, and glucose fermentation, and bacterial motility), as well as standard tests such as hot staining and the colony’s morphology and color assessment (20). The bacteria were then classified using Gram staining for the observation of Gram-negative cocci and diplococci and the oxidase test. In the next step, biochemical tests, culturing on mechanical agar, incubation at 37°C and 42°C, the citrate and motion tests, and fermentation (glucose, etc.) and oxidation analyses were performed to establish a definitive identification of the bacterium. In this study, for assessing the antibacterial effects of Eshvarak extracts, antibiotic-resistant bacteria were selected for further analyses.
3.3. Evaluation of Antibiotic Resistance
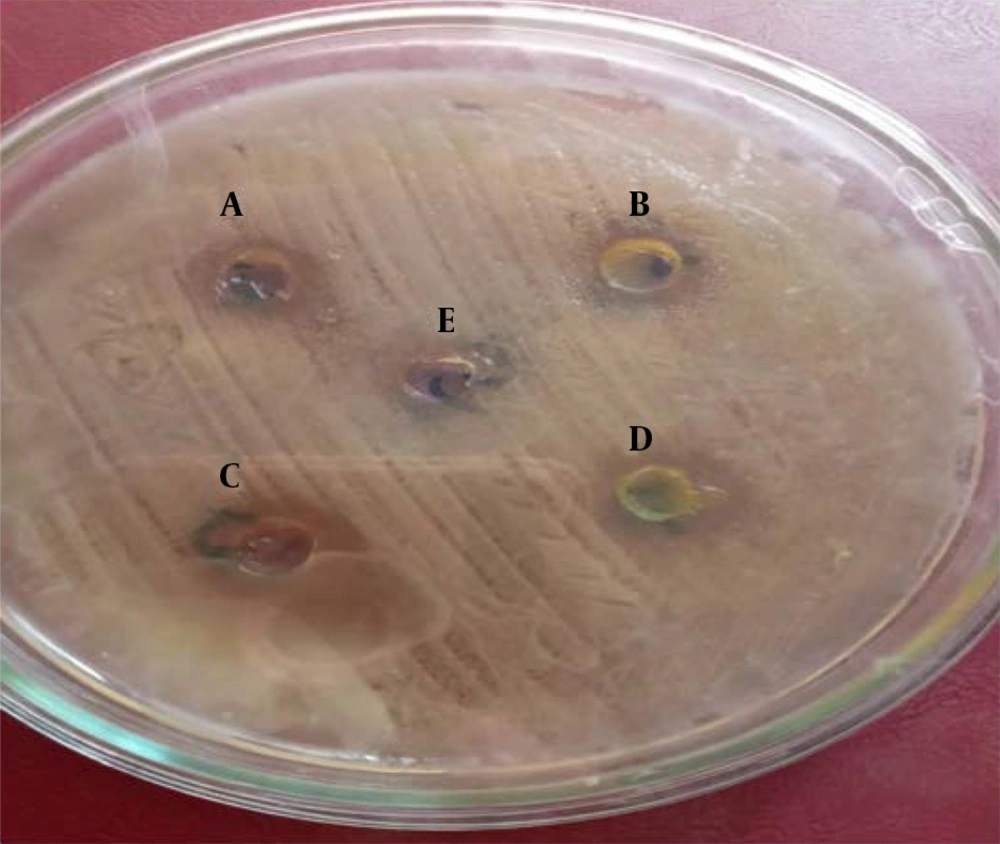
Ten pure E. coli isolates were identified by the Kirby-Bauer method (21), and their resistance to antibiotics was evaluated. For this, prepared bacterial suspensions were removed under sterile conditions next to a flame and a laminar hood and spread on the surface of a plate containing Muller Hinton agar. Next, four wells with diameters of 5 mm and a distance of 2 cm from each other were created on the plate’s surface. The prepared extracts with a concentration of 100 ppm were then added to each well and incubated at 37°C for 24 hours. After this, bacterial cultures were examined for the presence or absence of growth inhibition zones, and then the growth inhibition zone diameter was measured in millimeters by a caliper.
The sensitivity of the isolates to antibiotics such as gentamicin (GM) (10 μg), azithromycin (AZM) (15 μg), amoxicillin colloid acid (AMC) (30 μg), amikacin (AN) (30 μg), and cefazolin (CZ) (30 μg) (Iran) was determined (21). After 24 hours of incubation at 37° C, the diameter of the growth inhibition zone was measured for each antibiotic, and the results were recorded as sensitive, intermediate, and resistant according to relevant instructions and compared with the NCCLS standard table (22, 23).
3.4. Determination of the MIC and MBC of Plant Extracts Against Escherichia coli
To determine the minimum inhibitory concentrations (MIC) of plant extracts, 100 μL of Mueller Hinton Broth medium (Merk, Germany) was initially applied to each of a microtiter plate’s wells (24, 25). Then 100 μL of 20 mg/mL extract was added to the first well, and after mixing, 100 μL of the extract was removed from the first well and added to the second well. Double dilutions were continued in other wells. Using this method, the 20 mg/mL concentration of the plant extract was reduced to half in subsequent wells. Ten μl of each bacterial suspension (CFU = 1.5 × 108/mL, 0.5 McFarland) was applied to the wells. As the negative control, DMSO was applied to the well (without the plant extract). The microtiter plate was incubated at 37°C for 24 hours. The MIC was described as the lowest concentration needed to stop bacterial growth after 24 hours of incubation. To determine the minimum bactericidal concentration (MBC), 10 µL of the wells’ content was applied to the nutrient agar medium (Merk-Germany) at the end of the 24-hour incubation period. The lowest plant extract’s concentration at which 99.9% of bacteria did not grow was considered as MBC (24, 25). All antimicrobial tests were repeated three times.
4. Results
4.1. Methanolic Extract
The lowest inhibitory concentration of the plant’s methanolic extract was 12.5 ppm, at which the growth of two strains were suppressed. The highest inhibitory concentration of the plant extract was 50 ppm, at which one strain did not grow. The estimated MBC of the methanolic extract was 100 ppm, at which one strain was completely eradicated (Tables 1 and 2). The highest inhibitory zone diameter of the methanolic extract was 8 mm, recorded at the 100-ppm concentration; on the other hand, the lowest inhibitory zone diameter was 1 mm (Table 3 and Figure 2).
| Bacterial Strains | Extracts | ||||
|---|---|---|---|---|---|
| Methanolic | Aqueous | Hydroalcoholic | Ethanolic | Ethyl-acetate | |
| 1 | 12.5 | 25 | 25 | 50 | 50 |
| 2 | 25 | 50 | 50 | 50 | 25 |
| 3 | 25 | 25 | 50 | 50 | 25 |
| 4 | 50 | 25 | 25 | 25 | 50 |
| 5 | 25 | 50 | 25 | 25 | 12.5 |
| 6 | 25 | 50 | 25 | 25 | 25 |
| 7 | 25 | 12.5 | 50 | 25 | 50 |
| 8 | 25 | 12.5 | 25 | 25 | 25 |
| 9 | 25 | 12.5 | 25 | 25 | 50 |
| 10 | 12.55 | No growth | 25 | 12.5 | 50 |
| Bacterial Strains | Extracts | ||||
|---|---|---|---|---|---|
| Methanolic | Aqueous | Hydroalcoholic | Ethanolic | Ethyl-acetate | |
| 1 | 25 | 50 | 50 | 100 | 100 |
| 2 | 50 | 100 | 100 | 100 | 50 |
| 3 | 50 | 50 | 100 | 100 | 50 |
| 4 | 100 | 50 | 50 | 50 | 100 |
| 5 | 50 | 100 | 50 | 50 | 25 |
| 6 | 50 | 100 | 50 | 50 | 50 |
| 7 | 50 | 25 | 100 | 50 | 100 |
| 8 | 50 | 25 | 50 | 50 | 50 |
| 9 | 50 | 25 | 50 | 50 | 100 |
| 10 | 25 | No growth | 50 | 25 | 100 |
| Bacterial Strains | Extracts | ||||
|---|---|---|---|---|---|
| Methanolic | Aqueous | Hydroalcoholic | Ethanolic | Ethyl-acetate | |
| 1 | 8 | 5 | 5 | 7 | 5 |
| 2 | 3 | 8 | 8 | 0 | 5 |
| 3 | 1 | 2 | 3 | 2 | 0 |
| 4 | 5 | 6 | 5 | 6 | 1 |
| 5 | 1 | 3 | 2 | 3 | 0 |
| 6 | 5 | 2 | 3 | 4 | 0 |
| 7 | 5 | 3 | 1 | 2 | 0 |
| 8 | 5 | 6 | 8 | 5 | 5 |
| 9 | 5 | 6 | 8 | 5 | 5 |
| 10 | 5 | 8 | 10 | 6 | 3 |
4.2. Ethanolic Extract
The lowest inhibitory concentration of the ethanolic extract was 12.5 ppm, at which the growth of two strains were suppressed. The maximum inhibitory concentration of the extract was 50 ppm, which inhibited the growth of four strains. The highest MBC was 100 ppm, inhibiting the growth of four strains (Tables 1 and 2). The highest and lowest inhibitory zone diameters of the ethanolic extract were 7 mm and 2 mm, respectively (Table 3).
4.3. Aqueous Extract
The lowest MIC of the aqueous extract was 12.5 ppm, at which three strains were inhibited, and the highest MIC was 50 ppm, at which three strains were inhibited, and one strain was entirely eradicated. The highest MBC of the aqueous extract was 100 ppm, at which three strains were inhibited (Tables 1 and 2). The maximum and minimum inhibitory zone diameters of the aqueous extract were 8 mm and 2 mm, respectively (Table 3 and Figure 2).
4.4. Hydroalcoholic Extract
The lowest inhibitory concentration of the hydroalcoholic extract was 25 ppm, at which seven strains did not grow. On the other hand, at the MIC of the extract (50 ppm), the growth of three strains was inhibited. In comparison, the highest MBC was obtained 100 ppm, at which three strains were suppressed (Tables 1 and 2). The highest inhibitory zone diameter of the hydroalcoholic extract was 10 mm, and the lowest inhibitory zone diameter was 3 mm (Table 4 and Figure 2).
| Bacterial Strains | CZ | AMC | AZM | AN | GM |
|---|---|---|---|---|---|
| 1 | R | I | I | I | S |
| 2 | R | S | S | S | S |
| 3 | R | S | S | S | S |
| 4 | R | I | S | S | R |
| 5 | R | R | I | I | S |
| 6 | R | S | S | S | S |
| 7 | R | S | I | I | S |
| 8 | I | I | R | S | S |
| 9 | R | S | S | S | S |
| 10 | I | S | S | S | S |
4.5. Ethyl-acetate Extract
The lowest inhibitory concentration of the plant’s ethyl-acetate extract against E. coli was 12.5 ppm that inhibited the growth of one strain. The MIC was obtained as 50 ppm, at which five strains did not grow. The MBC of the ethyl-acetate extract was 100 ppm that suppressed the growth of five E. coli strains (Tables 1 and 2). The highest and lowest inhibitory zone diameters of the ethyl-acetate extract were 5 mm and 1 mm, respectively (Table 3). Regarding GM, AN, AZM, AMC, and CZ antibiotics, resistance rates were 10%, 0%, 10%, 10%, and 80%, and susceptibility rates were 90%,70%, 60%, 60%, and 0%, respectively (Table 4, Table 5 and Figure 2).
| GM | AN | AZM | AMC | CZ | |
|---|---|---|---|---|---|
| S | 90 | 70 | 60 | 60 | 0 |
| I | 0 | 30 | 30 | 30 | 20 |
| R | 10 | 0 | 10 | 10 | 80 |
5. Discussion
In the present study, we determined the lowest inhibitory concentrations of the methanolic (12.5 ppm), ethanolic (12.5 ppm), aqueous (12.5 ppm), hydroalcoholic (25 ppm), and ethyl-acetate (12.5 ppm) extracts of Eshvarak, as well as the highest inhibitory zone diameters of the methanolic (8 mm), ethanolic (7 mm), aqueous (8 mm), hydroalcoholic (10 mm), and ethyl-acetate (5 mm) extracts of the plant against E. coli.
The minimum inhibitory concentrations of Eshvarak ethyl-acetate extract have been assessed against standard bacteria such as Vibrio cholerae (20 mg/mL), E. coli (20 mg/mL), Pseudomonas aeruginosa (20 mg/mL), Bacillus cereus (10 mg/mL), and Shigella dysentery (10 mg/mL). Also, the minimum inhibitory concentrations of the plant’s hydroalcoholic extract have been described against standard V. cholerae (20 mg/mL), E. coli (10 mg/mL), P. aeruginosa (10 mg/mL), B. cereus (10 mg/mL), and S. dysentery (10 mg/mL). Finally, the lowest inhibitory concentrations of Eshvarak methanolic extract have been noted against V. cholerae (5 mg/mL), E. coli (5 mg/mL), P. aeruginosa (10 mg/mL), B. cereus (2.5 mg/mL), and S. dysentery (5 mg/mL) (12). In the present study, the methanolic extract of Eshvarak exerted the greatest inhibitory effects against E. coli.
In one study, the inhibitory zone diameters of Eshvarak methanolic extract against Trichophyton longifusis, Candida albicans, Fusarium solani were 25 ± 0.5, 23 ± 1, and 18 ± 1.5 mm; on the other hand, the inhibitory zone diameters of Eshvarak chloroform extract against T. longifusis, A. flavus, and M. Canis were 10 ± 0.5, 7 ± 1, and 11 ± 1.5 mm, respectively (26). In our study, the highest inhibitory zone diameter of Eshvarak methanolic extract against E. coli was 8 mm, which was obtained at the 100-ppm concentration.
The highest inhibitory zone diameter of Eshvarak aqueous extract against E. coli was 35 mm (17); however, the average inhibitory zone diameter of the aqueous extract was 10 mm. The antimicrobial properties of plant extracts are generally attributed to their phytochemicals and phenols; nevertheless, the amounts of phenolic and flavonoid compounds of various plant extracts vary based on the nature of plants and species, as well as the extraction method and the type of solvent (27). Therefore, it is suggested that depending on the purpose of an experiment, the effects of different plant species, the type of the solvent, and extraction methods be considered on the antimicrobial activity of herbal extracts.
In previous studies, methanol (28) and Acetone extract (29) have been suggested to be the most effective solvents for extracting the phenolic materials and preserving the oxidative properties of plants. In the present study, hydro-alcohol seemed to better preserve the phenolic materials of Eshvarak; however, this also depends on the extraction method.
The antibacterial effects of the methanolic extract of Carum copticum L. have been investigated in vitro against pathogenic Staphylococcus aureus, B. cereus, E. coli, and P. aeruginosa. The antibacterial effects of the methanolic extract of aloe vera were more pronounced against Gram-positive than Gram-negative bacteria. The highest inhibitory zone diameters were observed against S. aureus (19 mm) and B. cereus (14 mm). The MICs of aloe vera methanolic extract against S. aureus, B. cereus, and E. coli were 12.5 mg/mL, 25 mg/mL, and 50 mg/mL, respectively (30). In the present study, we found that the MIC of Eshvarak methanolic extract against E. coli was 12.5 mg/mL, suggesting that the antimicrobial capacity of Eshvarak extract against E. coli was higher than that of aloe vera.
Studying the inhibitory effects of the substances derived from weed, date palm, ash (Rhazya stricta Decne), fern (Ferula assa-foetida L.), and neem (Azadirachta indica A. Juss.), including alpha-pinene, eugenol, and thymol, on the spawning of Rhynchophorus ferrugineus showed that the lowest spawning was observed in exposition to thymol 10% (1.5 ± 0.54) and 10% ash leaf extract (1.6 ± 0.66). In addition, the highest inhibitory effects on ovulation were related to thymol 10% (85.63 ± 4.75%) and 10% Eshvarak extract (83.32 ± 7.70%). It has been proposed that thymol and ash extract can be used to cover wounds on date palm trees’ trunks and avoid the spawning of their weevils, minimizing new pest infestations (13). The antimicrobial properties of Eshvarak against E. coli were confirmed in this study, suggesting the beneficial effects of Eshvarak to treat E. coli infections; nevertheless, this needs to be assessed in clinical studies.
The effects of the extracts of starfish and mocha anemone obtained by diethyl-ether and methanol solvents on E. coli and B. subtilis were investigated. It was concluded that diethyl ether used to prepare anemone extract did not inhibit the growth of the studied bacteria. However, starfish-derived diethyl ether extract showed a growth inhibitory, but not lethal, effect against E. coli at the concentration of 40 mg/mL. These extracts did not affect the growth of B. subtilis at the studied concentrations. Asteroidea methanolic extract showed growth inhibitory (30 mg/mL) and lethal (50 mg/mL) effects against B. subtilis , but none of these concentrations had an effect on E. coli. Also, the methanolic extract of Stichodactyla haddoni at the concentration of 40 mg/mL inhibited E. coli growth but did not show a lethal effect; none of the concentrations tested had any effect on B. subtilis (31). In the present study, it was found that all Eshvarak extracts (methanolic, ethanolic, aqueous, hydroalcoholic, and ethyl acetate) had both inhibitory and lethal effects against E. coli, indicating the greater antimicrobial potency of Eshvarak extract compared with Asteroidea and S. haddoni against this bacterium.
The improper use of bactericidal agents in poultry breeding units without an accurate assessment of bacterial susceptibility leads to the development of antimicrobial-resistant bacteria and consequently the selection of more resistant bacterial clones, inflicting negative impacts on the poultry industry (32). Of course, this critical fact should not be overlooked that restricting the use of antibacterial agents may not necessarily reduce the frequency of drug-resistant infections. Therefore, it is controversial that resistance only occurs due to the pressure from the continuous use of antimicrobial agents, suggesting the possible role of other factors in the survival of drug-resistant organisms (33, 34).
In one study in Kermanshah (Iran), the E. coli isolated from poultry showed the highest antibiotic resistance against chloro-tetracycline, erythromycin, oxytetracycline, and cholestin during the first three months of the year. In addition to the above-mentioned antibiotics, a high resistance was reported against fluoxacine in summer (14). In a study in Isfahan province on the prevalence of antibiotic-resistant E. coli in chicken meat, the results showed that 20.45% of the samples were infected with E. coli. The bacterial isolates showed the highest resistance to gentamicin (84.44%), ampicillin (80%), ciprofloxacin (77.77%), endofloxacin (66.66%), and erythromycin (22.62%) antibiotics (15). On the one hand, in the present study, the resistance rates of E. coli against GM, AN, AZM, AMC, and CZ antibiotics were 10%, 0%, 10%, 10%, and 80%, respectively. Different used Eshvarak extracts inhibited the growth of these resistant E. coli strains, suggesting the plant as a suitable natural product to fight antimicrobial-resistant bacteria.
5.1. Conclusions
The antimicrobial properties of plant extracts are generally determined by the plant species, utilized extraction methods, and the type of solvent. In the present study, it was found that Eshvarak extracts, especially the hydroalcoholic extract, could inhibit the growth of antibiotic-resistant E. coli.