1. Background
In recent years, the prevalence of poisoning has dramatically increased due to communities' development and the ease of access to drugs and toxins. Arsenic compounds, as one of the common contaminants and toxins, are found inorganically and organically in the human body and environment (1) as arsenic poisoning has been reported to affect all body organs. Arsenic, especially inorganic arsenic, is absorbed through the gastrointestinal tract and spreads throughout the body (2), resulting in DNA damage, lipid oxidation, and decreased antioxidant levels of the defense system (1, 3).
Apoptosis is a natural cellular process regulating the balance between cell proliferation and death. This process initially compacts and fragmentizes the chromatin, amasses the cell cytoplasm, eventually leading to the crush of the nucleus and cell membranes and the production of vacuoles containing apoptotic particles (4). Different proteins play vital roles in regulating the apoptosis phenomenon, the most critical of which are Bcl-2 family proteins containing pro-apoptotic and anti-apoptotic factors that induce and inhibit apoptosis, respectively. Bcl-2 proteins maintain membrane integrity by binding channels to the mitochondrial outer membrane. However, an increase in the expression of Bax results in alterations in mitochondrial membrane permeability, the release of cytochrome C, apoptosis-inducing factors released from mitochondria, and eventually DNA fragmentation (5). Caspases are proteases acting as initiators and executioners of the apoptotic process (6). Caspase-3 is one of the main executioner proteases, which remains silent until activated by the initiator caspases through direct proteolysis (7, 8).
Regular aerobic exercise has been considered a fundamental factor in preventing cardiovascular disease over recent decades. Studies have revealed that regular exercise reduces cell apoptosis and that six weeks of low-intensity aerobic activity decreases apoptotic cells in the heart tissue of older rats (9). One study demonstrated the protective effect of 12-week moderate-intensity aerobic exercise on apoptosis by lessening caspase-9, caspase-3, and Bax to Bcl-2 levels in rats' heart tissue (10).
In addition to regular exercise training, medicinal plants have recently focused on treating and preventing many diseases. However, herbal supplements are less effective than synthetic drugs. In some cases, these herbal therapies are documented as an acceptable alternative to chemical drugs (11). In previous studies, pumpkin id one of the most useful herbs, whose traditional use has been confirmed. Pumpkin (Cucurbita pepo L.) is a one-year-old and crawling plant from the Cucurbitaceous family. Pumpkin is revealed to effectively treat many diseases such as digestive disorders, diabetes, liver disorders, kidney disease, cancer, and wound healing (12). Pumpkin seed has potential antioxidant activity due to phenolic compounds, tocopherols, and zinc. Moreover, a review of the literature revealed a high percentage of two unsaturated fatty acids, namely oleic acid (27 - 38%) and linoleic acid (43 - 55%), in its seeds (13). The antioxidant compounds in pumpkin have numerous health effects (e.g., preventing cardiovascular disease), and they also have anti-inflammatory properties (14).
2. Objectives
The present study aimed to investigate the simultaneous effects of regular aerobic exercise training and pumpkin seed extract on apoptotic factors such as Bcl-2, Bax, and Caspase-3 in the heart and aorta endothelial cells in rats poisoned with arsenic.
3. Methods
3.1. Animals and Ethics
In this study, 56 male Wistar rats aged 6-8 weeks old with 220-240 g weights were included. The animals were kept in an animal room at 19 - 22°C with a 12:12h light-dark cycle and free access to food and tap water. Animals were fed with a standard rodent laboratory diet (crude protein 19.50 - 20.50%, fat 3.5 - 4.5%, fiber 4 - 4.5%, calcium 0.95 - 1%, phosphorus 0.65 - 0.7%, salt 0.5 - 0.55%, lysine 1.15%, methionine 0.33%, threonine 0.72%, tryptophan 0.25%, and energy 16.16 - 17 mg/kg) and tap water. All animal experiments were approved by the Ethical Committee of Islamic Azad University, Arak Branch (Ethical number: IR.IAU.ARAK.REC.1398.012) and performed under the guidelines of National Institutes of Health (NIH).
3.2. Groups Category
Rats were randomly divided into seven groups (n = 8 per group), and were categorized based on receiving regular aerobic exercise and/or pumpkin seed extract consumption : (1) TC (toxic control), (2) AE (aerobic exercise training), (3) TAP1 (toxic aerobic exercise training + 300 mg/kg/day pumpkin seed extract consumption), (4) TAP2 (toxic aerobic exercise training + 600 mg/kg/day pumpkin seed extract consumption),(5) TP1 (300 mg/kg/day toxic pumpkin seed extract consumption), (6) TP2 (600 mg/kg/day toxic pumpkin seed extract consumption), and (7) HC (healthy control) groups.
The rats in all the groups (except for HC) were exposed to 25 ppm arsenic using sodium arsenite in their daily water for 16 weeks (15).
3.3. Pumpkin Seed Preparation (Crude Extract)
Iranian oil-free pumpkin seed (cold-pressed oil) was purchased from PAKAN BAZR Co., Isfahan, Iran. In the first step, 10 g of grinded seeds was mixed with 70% alcohol in a blender and incubated for 72 h at room temperature. Then ethanol liquid was separated with Whatman® Anotop® 10 syringe filters 0.02 μm paper. Finally, 300 and 600 mg/kg/day pumpkin seed extract were fed to rats by specific gavage (16).
3.4. Regular Aerobic Exercise Training Protocol
Firstly, all the rats got familiar with running on the treadmill with a 5% incline at a speed of 5 - 10m/min and 10 min/day for one week. Aerobic exercise training began with a warm-up and terminated with a cool-down, both of which consisted of 2 min running at 10 m/min. Then the main running started with 10 m/min, with a 5% incline for 10 min/day, so that the running speed and time were gradually increased up to 15m/min for 20 min/day for two weeks. In the last two sessions, aerobic activity intensity reached 25m/min for 30 min/day. Furthermore, the rats in the supplemental groups (groups 3 - 6) received 300 mg/kg/day and 600 mg /kg/day of pumpkin seed extract for eight weeks by oral gavage (17).
3.5. Tissue Collection
Rats were anesthetized by the intraperitoneal administration of a mixture of 30 - 50 mg/kg ketamine and 3 - 5 mg/kg xylazine 24 hours after the last intervention, during which they were on fast for 10 - 12 hours. Then the aortic endothelial and heart tissues were collected and homogenized at 4°C. After that,50 mg of the heart tissue was frozen in 1 mL of homogenized cellular hemoglobin buffer and then centrifuged for 1 minute at 1000 rpm. The supernatant was isolated,added to the protease inhibitor, and stored at - 80°C (18, 19).
3.6. Apoptosis Measurement
The standard Bradford method was used to measure total proteins. After preparing tissue homogenate, the levels of Bcl-2, Caspase-3, and Bax factors were measured using Zell Bio GmbH (ZB-10034C-R9648) ELISA kits, Germany, and analyzed by Hiperion MR4+ at 450 nm (20).
3.7. Statistical Analyses
Statistical analyses were performed using SPSS software version 22, USA. Shapiro–Wilk test results confirmed the normal distribution of the variables. Data were reported as means ± standard deviation (SD), and one-way ANOVA was used to detect the difference between the healthy-control (Non-intoxicated) and toxic–control groups. Moreover, two-way ANOVA was applied to examine the effectiveness of exercise training, supplement consumption, and a combination of exercise training and supplement consumption. Also, the effect size (partial η2) was reported to emphasize the size of the difference rather than confound the sample size (0.01, 0.03, and > 0.05 were considered small, medium, and large effects, respectively).
P < 0.05 was accepted as the significance level in this study.
4. Results
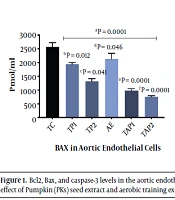
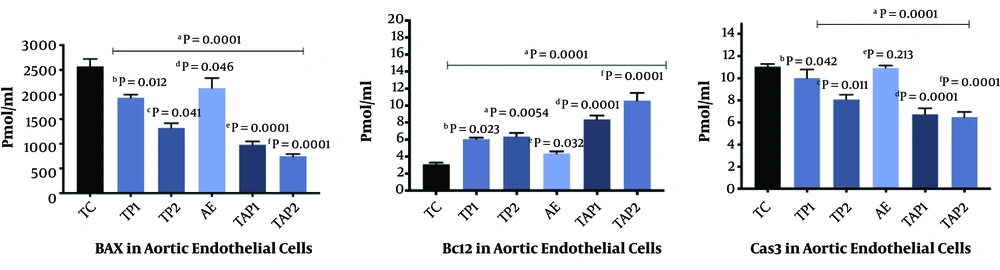
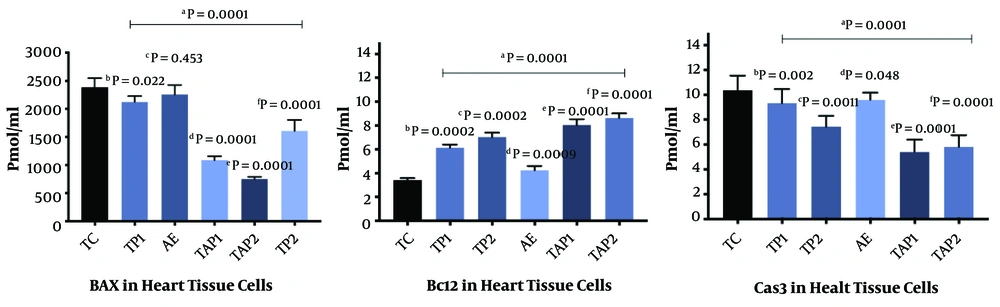
The findings of the present study indicated that the interventions significantly increased Bcl2 and decreased Bax and caspase-3 concentration in intoxicated rats (Table 1). As it is presented, in the aortic endothelial cells, aerobic exercise promotes Bcl2 (F = 6.46, P = 0.0001, Eta =0.28), and reduces Bax (F = 6.83, P = 0.0001, Eta = 0.29) and caspase-3 (F = 6.24, P = 0.0001, Eta = 0.28) (Figure 1). Moreover, the use of the supplement (i.e., pumpkin seed extract) triggered a significant increase in Bcl2 (F = 7.00, P = 0.0001, Eta = 0.29), and a decline in Bax (F = 6.88, P = 0.0001, Eta = 0.38) and caspase-3 (F = 6.86, P = 0.0001, Eta = 0.30). A combination of exercise training and pumpkin seed extract consumption augmented Bcl2 (F = 7.11, P = 0.0001, Eta = 0.29) and decreased Bax (F = 6.69, P = 0.0001, Eta = 0.31) and caspase-3 (F = 7.12, P = 0.0001, Eta = 0.32) (Figure 1). Regarding the changes in the heart tissue cells, exercise training increased Bcl2 (F = 6.49, P = 0.0001, Eta = 0.28) and decreased Bax (F = 7.23, P = 0.0001, Eta = 0.33) and caspase-3 (F = 6.23, P = 0.0001, Eta = 0.23) (Figure 2). Furthermore, pumpkin seed extract considerably raised Bcl2 (F = 7.77, P = 0.0001, Eta = 0.31) and reduced Bax (F = 6.88, P = 0.0001, Eta = 0.29) and caspase-3 (F = 6.23, P = 0.0001, Eta = 0.27) (Figure 2). Finally, a combination of exercise training and pumpkin seed extract consumption significantly increased Bcl2 (F = 6.99, P = 0.0001, Eta=0.36), and lowered Bax (F = 6.85, P = 0.0001, Eta = 0.33) and caspase-3 (F=7.33, P = 0.0001, Eta = 0.25) (Figure 2).
| Variables (Pmol/mL) | Healthy-Control | Toxic–Control | F-Value | P-Value | Eta |
|---|---|---|---|---|---|
| Endothelial aortic cells | |||||
| Bcl2 | 13.8 ± 0.5314 | 3.083 ± 0.1075 | 131.2 | < 0.0001 | 0.98 |
| Bax | 203.8 ± 16.75 | 2572 ± 88.22 | 180.7 | < 0.0001 | 0.98 |
| Cas 3 | 1.898 ± 0.1231 | 11.05 ± 0.1329 | 154.7 | < 0.0001 | 0.95 |
| Heart tissue cells | |||||
| Bcl2 | 12.74 ± 0.1501 | 3.421 ± 0.1017 | 245.0 | < 0.0001 | 0.99 |
| Bax | 295.4 ± 29.91 | 2387 ± 92.69 | 124.6 | < 0.0001 | 0.98 |
| Cas 3 | 1.612 ± 0.1441 | 10.38 ± 0.1333 | 32.75 | < 0.0001 | 0.93 |
aData Are Presented as Mean ± SD (N = 8 Per Group)
Bcl2, Bax, and caspase-3 levels in the aortic endothelial cells in different groups. Each group consists of 8 rats, and data are expressed as mean ± SD. a Significant effect of Pumpkin (PKs) seed extract and aerobic training exercise in the case groups vs. control group. b TP1 vs. TC. c TP2 vs. TC. d AE vs. TC. e TAP1 vs. TC. f TAP2 vs. TC.
Bcl2, Bax, and caspase-3 levels in the heart tissue cells in different groups. Each group consists of 8 rats, and data are expressed as mean ± SD. a Significant effect of Pumpkin (PKs) seed extract and aerobic exercise training in the case groups vs. control group. b TP1 vs. TC. c TP2 vs. TC. d AE vs. TC. e TAP1 vs. TC. f TAP2 vs. TC.
The results also indicated that pumpkin seed extract at the concentrations of 300 and 600 mg/kg (with no aerobic exercise consumption) significantly increased Bcl-2 expression in both tissues (Figures 1 and 2). In contrast, aerobic exercise training could make no significant change in Bcl-2 when administered alone. Notably, when a combination of both interventions were used, the Bcl2 levels were more saliently augmented in the aorta endothelial and heart tissues (Figures 1 and 2). Moreover, the findings about pro-apoptotic factor Bax suggested that pumpkin seed extract had a significant effect on Bax at both concentration levels in the aorta endothelial (Figure 1); however, only a high concentration of the herbal extract (600 mg/kg) significantly decreased Bax in the heart tissues (Figure 2). Moreover, a single prescription of aerobic exercise had a statistically effective impact only on the aorta endothelial but not on the heart tissues. Similarly, the enhanced alterations were observed in Bax in the groups receiving both pumpkin seed extract and aerobic exercise training simultaneously (Figures 1 and 2).
Finally, the results revealed that pumpkin seed extract downregulated the pro-apoptotic factor caspase-3 only at high concentrations, and aerobic exercise did not decrease the given factor when applied to rats without plant extract. Likewise, a more significant decrease of caspase-3 was noticed in the groups receiving both pumpkin seed extract and aerobic exercise training.
5. Discussion
One of the significant health problems of today's societies is poisoning with metals such as arsenic and their accumulation in the food chain as these compounds cause serious damage to body tissues by increasing the production of free radicals (21). The myocardium is highly vulnerable to ROS free radicals due to high oxygen consumption and flawed antioxidant systems. Higashi et al. (2009) showed that ROS plays a vital pathophysiological role in cardiac patients, and that different ROS induce apoptotic signaling pathways within myocardial cells (22). Hydrogen peroxide (H2O2) is one of the most potent ROSs, which pose serious damage to the myocardial sarcolemma. It also plays a salient role in inducing apoptosis by affecting mitochondria (23). By increasing ROS, arsenic poisoning reduces mitochondrial membrane potential, which triggers the cytochrome C release into the cytoplasm, procaspase-9 activations, and apoptosis induction by transmitting the message to the executive caspases such as 3, 6, and 7 (24).
Preventing damages by apoptosis in myocardial cells is of great importance, and, over the past few decades, researchers have reported that regular aerobic exercise can reduce apoptosis in cardiac cells (25). As a mechanism, physical activity seems to be partially beneficial in reducing myocardial apoptosis by decreasing ROS production and preventing subsequent discharge of mitochondrial cytochrome C (26). According to previous studies, aerobic exercise is noticeably beneficial in reducing myocardial apoptosis by decreasing ROS and preventing mitochondrial cytochrome C release. Kanter et al. (2017) also implied that aerobic exercise significantly lowered oxidative stress and apoptosis; however, it heightened antioxidant activity in individuals’ hearts (27). Other mechanisms associated with the effect of aerobic exercise training on apoptosis have addressed the effect of the exercise on several molecules in the mitochondrial pathway and apoptosis cascade. The mitochondrial pathway of apoptosis is mainly mediated by Bcl-2 family proteins, which inhibit mitochondrial cytochrome C release (28). Santana et al. (2014) examined the effect of aerobic training on the expression of anti-apoptotic and pro-apoptotic proteins and found out that aerobic training decreased the expression of pro-apoptotic proteins such as Bad and increased the expression of anti-apoptotic proteins, includingBcl-2, c-IAP1, and c-IAP2 (29).
Moreover, Lai et al. (2014) stated that aerobic exercise training inhibited myocardial apoptosis by activating specific signaling pathways. The researchers also note that aerobic exercise often uses the SIRT / PGC-1α signaling pathway to inhibit apoptosis rather than the IGF1R / PI3K / Akt pathway (30). In their study, Akbari et al. (2017) showed that the increased production of free radicals raises cardiomyocyte apoptosis, and regular aerobic exercise led to prosperous changes in cell apoptosis via changes in the expression of anti-apoptotic and pro-apoptotic genes. Finally, exercise can decrease cardiomyocyte apoptosis by reducing the expression of the caspase-3 gene significantly (31).
On the other hand, pumpkin seed extract can dwindle hypertension and has anti-inflammatory effects decreasing the prevalence of various risk factors associated with cardiovascular diseases such as hyperlipidemia. Containing antioxidant compounds, Pumpkin also prevents lipid peroxidation, free radical production, and tissue damage .
The findings of the present study confirmed the decrease of Bax and caspase-3 and the increment of Bcl-2 protein, suggesting the anti-apoptotic effects of regular aerobic exercise alongside pumpkin supplementation in cardiac tissue. In other words, the study revealed an increase in Bax to Bcl-2 ratio because of increased ROS production in cardiac tissue caused by arsenic poisoning, which leads to the release of cytochrome C, the activation of caspases cascade, the activation of caspase-3, and ultimately DNA cleavage and cell death. In contrast, a combination of regular exercise and pumpkin supplementation reduced caspase-3 levels and decreased Bax to Bcl-2 ratio in the cardiac and aortic endothelial cells. Accordingly, it can be concluded that the combination of these two interventions leads to reduced apoptosis by suppressing the mitochondrial pathway; therefore, a reduction in Bax to Bcl-2 ratio due to regular exercise and pumpkin supplementation may decrease the expression of caspase-3 in the heart and aortic endothelial cells. Razavimajd et al. (2016) investigated the simultaneous effects of regular aerobic exercise and garlic extract on some apoptotic regulatory factors in old rats with chronic kidney diseases. In their study, eight weeks of swimming training, garlic supplementation, and a combination of both interventions increased renal Bcl-2 levels in the rats. Moreover, the renal levels of the Bax factor and Bax to Bcl-2 ratio significantly decreased in the rats after eight weeks of swimming training, garlic supplementation, and combined therapies (32). In agreement with our findings, Akbari et al. (2018) assessed the concurrent effect of six-week swimming and crocin supplement on the expression of the caspase-3 gene in rats’ cardiomyocytes infected with H2O2. Their findings revealed that H2O2 significantly increased caspase-3 gene expression, and six weeks of swimming resulted in a significant downregulation of caspase-3 in rats’ myocardial tissues (31). Moreover, Norouzi Kamareh et al. (2018) found that a combination of 12 weeks of aerobic training and green tea extract significantly decreased caspase-3 expression in aged male rats.
In this study, we examined the effect of regular aerobic exercise and pumpkin seed extract on the apoptosis of the heart and aortic endothelial cells. The findings show that the consumption of pumpkin seed extract or regular aerobic exercise reduces the pro-apoptotic factors Bax and Caspase-3. However, their concurrent administration significantly reduces these proteins in the heart tissue cells and aortic endothelial cells. Furthermore, the synchronous usage of regular aerobic exercise training and pumpkin seed extract, especially at a dose of 600 mg/kg, significantly increases the anti-apoptotic factor Bcl-2 protein in both tissues. One of the strengths of the present study is the measurement of key gene expressions related to apoptosis. However, the present study used no histological image; hence, future researchers are recommended to include histological images into future studies.
5.1. Conclusions
According to the findings, although both aerobic exercise training and pumpkin seed extract consumption reduce the apoptosis rate and thus have protective effects on heart tissue, the concurrent prescription of both interventions can significantly reinforce the effectiveness of another one in this protection.