1. Background
Investigating the antimicrobial effects of secondary metabolites of medicinal plants, such as essential oils and plant extracts, showed that at least one-third of all medicinal products are of plant origin or have been modified after extraction from the plant. In addition to preventing the growth of bacteria and food contaminated mold, these substances are used to increase the shelf life of processed foods as well as fruits and vegetables (1-7). Chicory (Cichorium intybus L.) belongs to the genus Asteraceae. Its roots, leaves, and seeds contain several medicinal compounds such as inulin, sesquiterpene, Lacoten, Kumarin, flavonoids, and vitamins that are used as anti-hepatitis, anti-nephrotic, anti-cancer, appetizer, and anti-inflammatory. In addition, their antibacterial properties against chicory root extract have been reported (8-10).
Hypericum perforatum L. belongs to the genus Clasias and contains a wide range of secondary metabolites such as flavonoids, proanthocyanidins, and naphthodiandrons (ipricin and pseudoeprycin (acyl fluoroglucinols (hyperforin and adheiphorphin)). It has many therapeutic properties like hypothyroidism, anti-inflammatory, anti-viral, antioxidant, anti-tumor, and antibacterial, but its most significant effect and application has been reported in the treatment of depression (11-13).
Lavandula angustifolia not only affects most organs and cells of the body but also has analgesic and anti-inflammatory effects and can reduce morphine tolerance and dependence. Lavandula angustifolia also affects cellular mechanisms such as oxidation reactions (reduction of oxidative reactions), programmed cell death (anti-apoptosis), and nitric oxide production (reduction of NO) and can affect cell genetic health. Lavandula angustifolia seems to be exerted by calmodulin calcium and its related kinases (14, 15).
Thymus vulgaris L. is a perennial plant of the genus Lamiaceae (Labiatae) with a cushion or clumpy structure. This plant is native to the countries of the Mediterranean region and grows in arid areas and between the boulders of the Mediterranean region, especially in France, Spain, Portugal, and some parts of Asia. Thymus vulgaris L. contains essential oils and compounds such as flavonoids, saponins, and bitter substances. The most critical components of Thymus vulgaris L. essential oil are phenolic compounds like thymol and carvacrol (5, 7).
The Taxus baccata L. is a coniferous leaf belonging to the Taxaceae family. Its medicinal value roots in the presence of Paclitaxel under the brand name Taxol in its needle leaves. Taxol, by forming an abnormal division spindle, can cease DNA transcription at the G2/M stage of mitotic division, thereby killing proliferating cells. The Food and Drug Administration has approved Taxol in 1977 for the treatment of uterine and breast cancers. Despite the invention of new methods of preparation of Taxol, such as cell culture, extraction from plant sources still retains its importance and place in supplying this valuable drug (16, 17).
As different medicinal plants pose various effects on microbes and the other hand the use of antibiotics is common (18).
2. Objectives
The current study aimed to investigate the effects of Cichorium intybus L., Hypericum perforatum L., Lavandula angustifolia, Thymus vulgaris L., and Taxus baccata on the stunting and non-growth of Bacillus cereus standard bacteria (i.e. Escherichia coli, Shigella dysentery, and Pseudomonas aeruginosa) as well as the clinical fungi (i.e. Candida albicans 1, Candida albicans 2, Candida albicans 3 and Candida albicans 4).
3. Methods
3.1. Herbal Materials
Cichorium intybus L., Hypericum perforatum L., Lavandula angustifolia, and Thymus vulgaris L. from Shahrekord (Coordinates: 32°19’32”N 50°51’52 E) and the leaves of the yew tree (Taxus baccata L.) were collected from Behshahr, Mazandaran province (36°41’32 36 N°53°33 53 09’E), and the species were determined in the botanical laboratory of the University of Zabol.
3.2. Method of Preparing Ethanolic Extract
The leaves of the plants used were dried in the shade and in the vicinity of air. Then 40 grams of leaves were ground (IKA company model A11 basic in Germany). The dried leaves were then soaked in 400 cc of ethanol for 96%. Shake for 48 hours at room temperature on a shaker (UniEquip SKIR-601L Germany). Then, the extracts were filtered, and the solvent was evaporated at a temperature of less than 40°C by a rotary device (Pars Azma Company, model RO02, Iran). The weighted extracts were weighed, and then 100 mg of the extract powder was dissolved in 1 cc of DMSO solvent. The extracts were stored in the refrigerator at 4°C until used in antimicrobial experiments (5).
3.3. Bacterial and Fungal Strains
Standard strains of bacteria such as Bacillus cereus (ATCC117718), Escherichia coli (ATCC700728), Shigella dysentery (ATCC13313), and Pseudomonas aeruginosa (ATCC27853) were obtained from Patten Teb. Also, clinical fungi of Candida albicans 1, Candida albicans 2, Candida albicans 3, and Candida albicans 4 were prepared from female patients in Zabol City.
3.4. Investigation of Antimicrobial Effects
In this section, the antimicrobial effects of the abovementioned plant extracts on human pathogens were investigated by diffusion method in Müller Hinton agar culture medium (made by Merck Germany) using paper discs (6 mm). In addition, microbial susceptibility was determined following the method proposed by Bauer et al.
3.5. Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
To determine the minimum inhibitory concentration (MIC) of essential oils of plants used by eye method, the first 100 microliters of Mueller Hinton broth (made by Merk-Germany) was added to each well of the titer plate (19, 20). Then, 100 µL of dilution of 20 mg/mL of essential oil was added to the first well, followed by mixing. In the next step, 100 µL of the first well was removed and added to the second well. Similarly, the construction of double dilutions in other wells was continued. It should be noted that in this case, the first well contained 20 mg per microliter of essential oil, and, thus, in subsequent wells, its concentration was half of the previous well. Eventually, 10 µL of each bacterial suspension (cfu = 108 1 1.5 per mL, half McFarland) was added to the wells. DMSO was added to the negative control well (without essential oil), followed by incubation at 37°C for 37 hours.
The MIC was defined as the lowest concentration required to stop the bacterial growth after 24 hours of incubation. To determine the minimum bactericidal concentration (MBC), 10 microliters of the wells was secondarily cultured on a nutrient agar medium (manufactured by Merk-Germany) after 24 h of incubation, and plates were examined for bacterial growth for 24 hours. The lowest concentration of essential oil in which 99.9% of bacteria did not grow was considered as MBC (19, 20). All antimicrobial tests were performed in triplet.
3.6. Data Analysis
Statistical calculations were administered using Statistix software version 10. Mean comparisons were performed using the least significant difference (LSD) at the 1% level, and Excel was also used to draw the shapes.
4. Results
4.1. Diameter of Inhibition Zone of Plant Extracts on the Bacteria Used
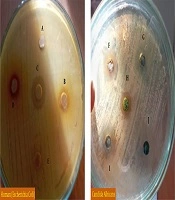
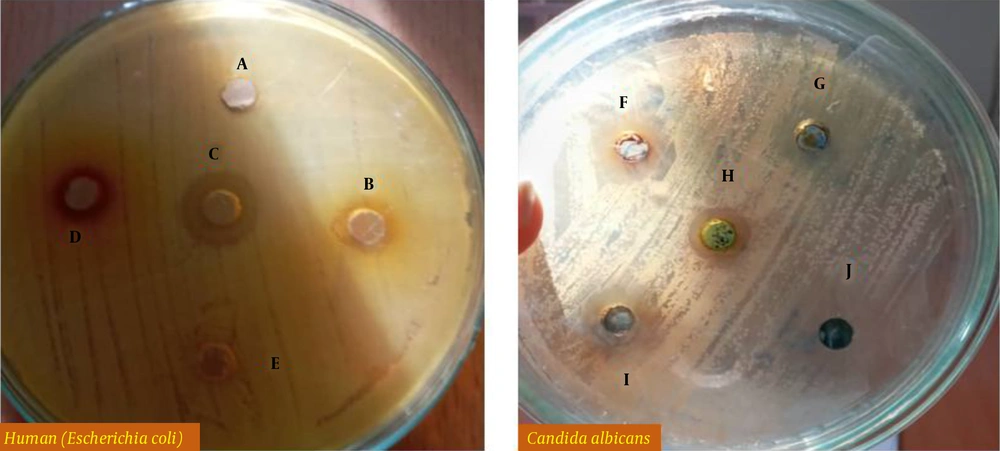
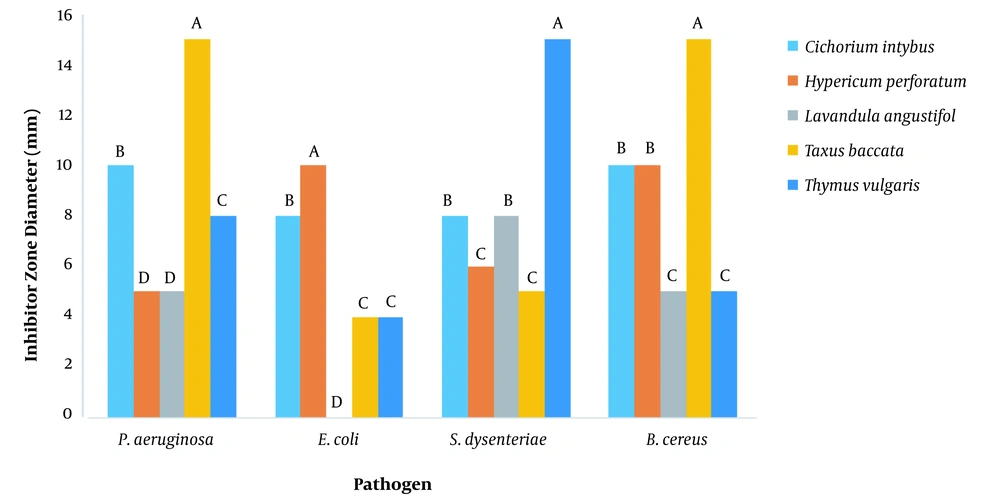
The inhibitory diameter zone of plant extracts against different bacteria was diluted to 100 ppm, and it was found that different extracts had different effects on growth inhibition of Bacillus cereus, Escherichia coli, Shigella dysentery, and Pseudomonas aeruginosa (P < 0.01) (Figure 1 and Table 1). According to the LSD post hoc test, the yew tree, with a 15 mm diameter, growth inhibition zone was the most useful plant concerning inhabitation of Bacillus cereus and Pseudomonas aeruginosa growth. Thymus vulgaris L., with a diameter of 15 mm, was not the most useful plant in inhibiting the growth of Shigella dysentery. With a diameter of 10 mm, it was not the most useful plant in inhibiting the growth of Escherichia coli (Figure 2).
| Source | DF | SS | MS | F |
|---|---|---|---|---|
| Plant | 4 | 207.60 | 51.9 | 51.9a |
| Error | 10 | 10 | 1.00 | |
| Total | 14 | 217.60 |
aSignificant at the level of one percent.
4.2. Diameter of Inhibition Zone of Plant Extracts on Candida albicans
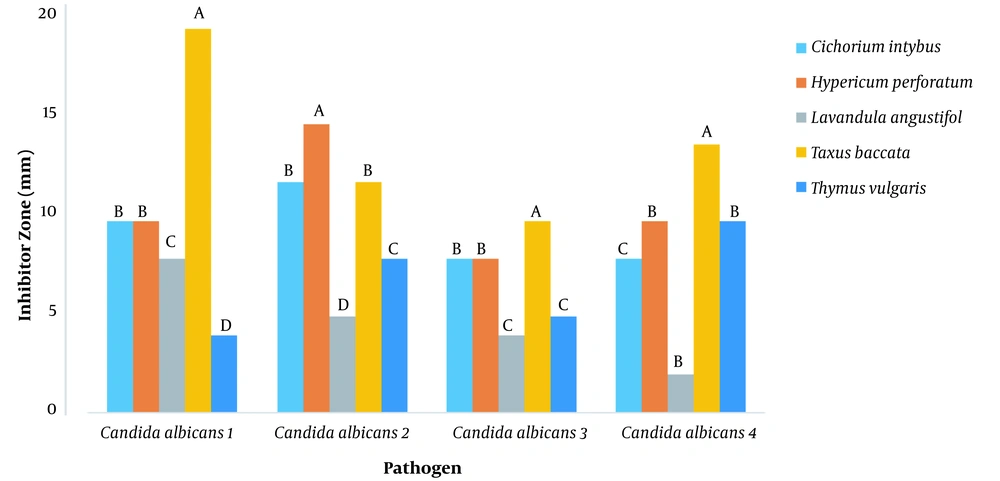
The diameter of the inhibitory zone diameter of plant extracts against Candida albicans at 100 ppm was investigated and it was found that different extracts had different effects on inhibiting the growth of Candida albicans (P < 0.01) (Figure 1 and Table 2). LSD post hoc test showed that the yew tree with 20-, 10-, and 14-mm diameter growth inhibition zone was the most useful plant on Candida albicans 1, 3, and 4, respectively. Herring flower was the most useful plant against Candida albicans 2 (a diameter of 15), followed by the yew tree with yew and chicory (diameters of 12 and 12, respectively) (Figure 3).
| Source | DF | SS | MS | F |
|---|---|---|---|---|
| Plant | 4 | 417.6 | 104.4 | 163.12a |
| Error | 10 | 6.4 | 0.64 | |
| Total | 14 | 424.00 |
aSignificant at 1%
4.3. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of Plant Extracts
The MIC of ethanolic extract of yew on Bacillus cereus, Escherichia coli, and Pseudomonas aeruginosa was 12.5, 12.5, and 25 ppm, respectively; in contrast, Escherichia coli was inhibited in all concentrations of yew and the lowest lethal concentration on yew Bacillus cereus, Escherichia coli, and Pseudomonas aeruginosa was 25 - 25 and 50 ppm, respectively (Table 3). The MIC of chicory ethanolic extract against Bacillus cereus, Escherichia coli, Shigella dysentery, and Pseudomonas aeruginosa was 25 - 25 - 50 and 25 ppm, respectively, and the highest lethal concentration was 100 ppm when Shigella dysentery was killed (Table 3). The MIC of ethanolic extract of Thymus vulgaris L. against Bacillus cereus, Escherichia coli, Shigella dysentery, and Pseudomonas aeruginosa was 25 - 50 - 25 and 12.5 ppm, respectively, and the highest lethal concentration against Bacillus cereus, Shigreshi, Escherichia, Pseudomonas aeruginosa was equal to 50, 100, 50, and 25 ppm (Table 3).
| Bacteria | Cichorium intybus L. MIC/MBC | Thymus vulgaris L MIC/MBC | Hypericum perforatum L. MIC/MBC | Nepeta binaludensis Jamzad MIC/MBC | Taxus baccata L. MIC/MBC |
|---|---|---|---|---|---|
| Bacillus cereus | 25 - 50 | 12.5 - 25 | 25 - 50 | 25 - 50 | 12.5 - 25 |
| E. coli | 25 - 50 | 50 - 100 | 50 - 100 | 25 - 50 | No growth |
| Shigella dysenteriae | 12.5 - 25 | 50 - 100 | 25 - 50 | 50 - 100 | 12.5 - 25 |
| Pseudomonas aeruginosa | 12.5 - 25 | 50 - 100 | 12.5 - 25 | 25 - 50 | 25 - 50 |
The MIC of Hypericum perforatum L. extract against Bacillus cereus, Escherichia coli, Shigella dysentery, and Pseudomonas aeruginosa was 12.5, 50, 50, and 50 ppm, and the MBC was 25 - 100 - 100 - 100. The minimum inhibitory concentration of Lavandula angustifolia extract against bacteria was 12.5 - 25, and 12.5 ppm, and the MBC was 25 - 50 - 50 and 25 ppm (Table 3).
Investigating the antifungal activity of plant extracts showed that the lowest inhibitory concentration of the yew extract was equal to 12.5 ppm, which was inhibited by both parties, in comparison, the lowest inhibitory concentration of chicory extract was 25 ppm, which all strains were inhibited, and the lowest inhibitory concentration of Thymus vulgaris L. was 12.5 ppm, of which 3 strains were inhibited (Table 4). The lowest and highest inhibitory concentrations of basil ethanolic extract against Candida albicans were 25 and 100 ppm, respectively. The MIC of Lavandula angustifolia extract against Candida albicans was 25 ppm, in which all strains were inhibited (Table 4).
| Spices | Cichorium intybus L. MIC/MBC | Thymus vulgaris L MIC/MBC | Hypericum perforatum L. MIC/MBC | Nepeta binaludensis Jamzad MIC/MBC | Taxus baccata L. MIC/MBC |
|---|---|---|---|---|---|
| Candida albicans 1 | 25 - 50 | 50 - 100 | 12.5 - 25 | 25 - 50 | 25 - 50 |
| Candida albicans 2 | 25 - 50 | 100 - 200 | 12.5 - 25 | 25 - 50 | 12.5 - 25 |
| Candida albicans 3 | 25 - 50 | 50 - 100 | 25 - 50 | 25 - 50 | 25 - 50 |
| Candida albicans 4 | 25 - 50 | 25 - 50 | 12.5 - 25 | 25 - 50 | 12.5 - 25 |
5. Discussion
According to the findings, the yew tree with a growth inhibition zone of a 15 mm diameter was the most useful plant to inhibit the growth of Bacillus cereus and Pseudomonas aeruginosa. Thymus vulgaris L., with a diameter of 15 mm, was not the most useful plant in inhibiting the growth of Shigella dysentery. With a diameter of 10 mm, this herb was the most useful plant in inhibiting Escherichia coli. The yew tree with a growth inhibition zone of 20-, 10-, and 14-mm was the most useful plant on Candida albicans 1, 3, and 4, respectively. Herring flower was the most useful plant against Candida albicans 2 (a diameter of 15), followed by the yew tree with yew and chicory (diameters of 12 and 12, respectively).
A study on the chemical composition and antibacterial activity of Iranian Lavandula × hybrid concluded that the diameter of the growth inhibition zone against S. aureus and E. coli was 9.36 and 23.3 mm, respectively. A significant association between the composition of essential oil and the level of antibacterial effect, expressed as inhibition areas, has been reported (21). In the present study, no E. coli was grown in a medium containing N. binaludensis Jamzad extracts.
A study on antibacterial properties of essential oils and hydrosols and aqueous extracts of Lavandula spp. grown in Australia reported that the hydrosols and aqueous extracts of the leaves of the plant had no antibacterial activity. This study also and concluded that different species of Lavandula spp. may have different antibacterial properties (22). In the present study, N. binaludensis Jamzad was effective against E. coli and no E. coli was grown, which confirms the different effects of various species of lavender against bacteria. They have interesting microbes and maybe a new potential source of natural antimicrobial as well as a new wound healing product (23), which confirms the effect of plant species on antimicrobial properties. The synthesis of antibacterial silver nanoparticles has been investigated using the extract of yew (Taxus baccata L.). It was concluded that the lowest MIC was obtained for S. pyogenes at a concentration of 50 µg/mL and for E. coli and S. aureus at a concentration of 25 µg/mL. Finally, they suggested that the use of biological compounds, mainly plant extracts, instead of toxic and hazardous chemicals, to synthesis silver nanoparticles, could reduce the environmental concerns regarding these nanoparticles (24). Similar to the findings of the present study, the MIC of ethanolic extract of yew on Bacillus cereus, Escherichia coli, and Pseudomonas aeruginosa was 12.5, 12.5, and 25 ppm.
The antimicrobial effects of watercress extract and the derived micro-emulsion on gram-negative clinical strains were investigated, and the highest zone and micro-emulsion were obtained at a concentration of 8 mg/mL with a diameter of 42 mm. This composition formed a zone with a diameter of 30 mm on the same bacterium at a concentration of 0.1 mg/mL; however, no zone formation was observed on Pseudomonas. The highest effect of the extract, which resulted in the MIC, was observed at 0.01563 mg/mL on Salmonella and for Shigella 0.00625 mg/mL. The MIC of the extract was 0.5 mg/mL for Escherichia coli and Proteus and 0.025 mg/mL for micro-emulsion of both bacteria (25). In the present study, the largest diameter of the growth inhibition zone against Candida albicans was 20 mm.
Medicinal plants have special place in the field of allochemicals, mainly due to their secondary metabolites. In addition, the demand for medicinal compounds has increased, but some of these plants have limited natural habitats, and depending on the environmental and geographical conditions, their collection may be difficult. Researchers have focused on the use of biotechnology techniques to increase the production and productivity of medicinal plants as follows; Low concentration of phytochemicals in plants, limitation of natural resources, increasing degradation of forests, pastures, and green space, extinction of diverse plant species, problems related to domestication and crop cultivation. Biotechnology can provide solutions to increase the efficiency of producing medicinal plants, as renewable sources for drug production, using various sciences such as biology, biochemistry, genetics, etc., and using cell, organ, and porcine culture methods as well as genetic engineering, and molecular markers (8).
Overuse of antibiotics has often led to the growing resistance of bacteria to these drugs. On the other hand, overuse of antibiotics is often associated with side effects in the human body. Because some plants have antimicrobial properties, they can be used to fight specific pathogenic microbes and find harmless alternatives to antibiotics.
On the other hand, in recent years, the effectiveness of antibiotics has decreased as a result of microbial resistance that led to the growth of research intended to develop new antibiotic compounds or to enhance the performance of antibiotics, among which increased attention has been paid to medicinal plants (26-28). Hence, the plants used in the present study can be examined against other microbes to obtain a comprehensive result. However, further studies are needed to determine the best-uncomplicated dose with the highest effectiveness as well as the active ingredient.
5.1. Conclusions
Despite their widespread benefits, chemical drugs, including antibiotics, also have side effects. According to the findings of the present study, the yew tree (Taxus baccata L.) was the most useful plant against Candida albicans, Bacillus cereus, and Pseudomonas aeruginosa. Hypericum perforatum L. was also the most useful plant in controlling the growth of Escherichia coli.