1. Background
Trisomy 21, called Down syndrome (DS), is one of the most common clinical syndromes, with a prevalence of one in every 800 - 1000 live births (1). The syndrome is a common genetic cause of intellectual disabilities worldwide, and many DS patients around the world face a variety of additional health issues, including heart defects, hematopoietic disorders, and early-onset Alzheimer disease (2). Furthermore, because babies with congenital anomalies place significant economic, social, and cultural burden on families and societies, the early detection of such defects during pregnancy can prevent the birth of children with disabilities (3). First-trimester screening (FTS), a combination of tests including fetal crown-rump length (CRL), nuchal translucency (NT) thickness, maternal serum free β-human chorionic gonadotropin (β-hCG), and pregnancy-associated plasma protein-A (PAPP-A), is one of the preventive programs adopted as the first-line screening method for DS in many countries (4), with a detection rate of 84%-90% and a false positive rate of 5% (5). However, it is unclear if the wide DS detection rates published in various studies can be applied universally to women of different ages. The effectiveness of FTS in women of different ages has not been adequately studied, suggesting a lower effectiveness in women under 35 years of age (6). The age of 35 years is considered as a threshold in this condition, and until the mid-1980s, prenatal diagnostic tests were only recommended for women older than 35 years. The incidence of DS has been reported to have a direct linear relationship with maternal age, and its prevalence progressively increases at the age of 40 years and over (7). However, about 80% of children with DS are born to mothers under 35 years of age (8).
2. Objectives
The present study was performed to evaluate the performance and effectiveness of combined FTS for DS based on maternal age in Southwest Iran.
3. Methods
3.1. Subjects
In this cross-sectional study, a prospective screening was performed to assess the risk of trisomy 21 during the first-trimester of pregnancy, according to the guidelines established by the Fetal Medicine Foundation (FMF) and based on a combination of fetal CRL, NT thickness, PAPP-A, and β-hCG measurement. The screening was conducted from the 11 + 0 to 13 + 6 weeks of gestation on 7192 pregnant women with different ages, referred for prenatal care to the Narges Medical Genetics and Prenatal Diagnosis Laboratory in Ahvaz, Southwest Iran, during May 2014 to March 2015. This study was approved by the Ethics Committee of the Biology Department, Shahid Chamran University of Ahvaz, Iran (no.: 20.6.505). All patients were informed of study aims and protocols and signed a written informed consent. Inclusion criteria included being in the first trimester of pregnancy and filling out an informed consent form. Gestational age, maternal age, weight, parity, polygamy, a history of giving birth to newborns with congenital anomalies, smoking, alcohol consumption, and a history of receiving treatments were recorded in a questionnaire.
3.2. Assessing Fetal CRL, NT Thickness, Maternal PAPP-A, and β-hCG
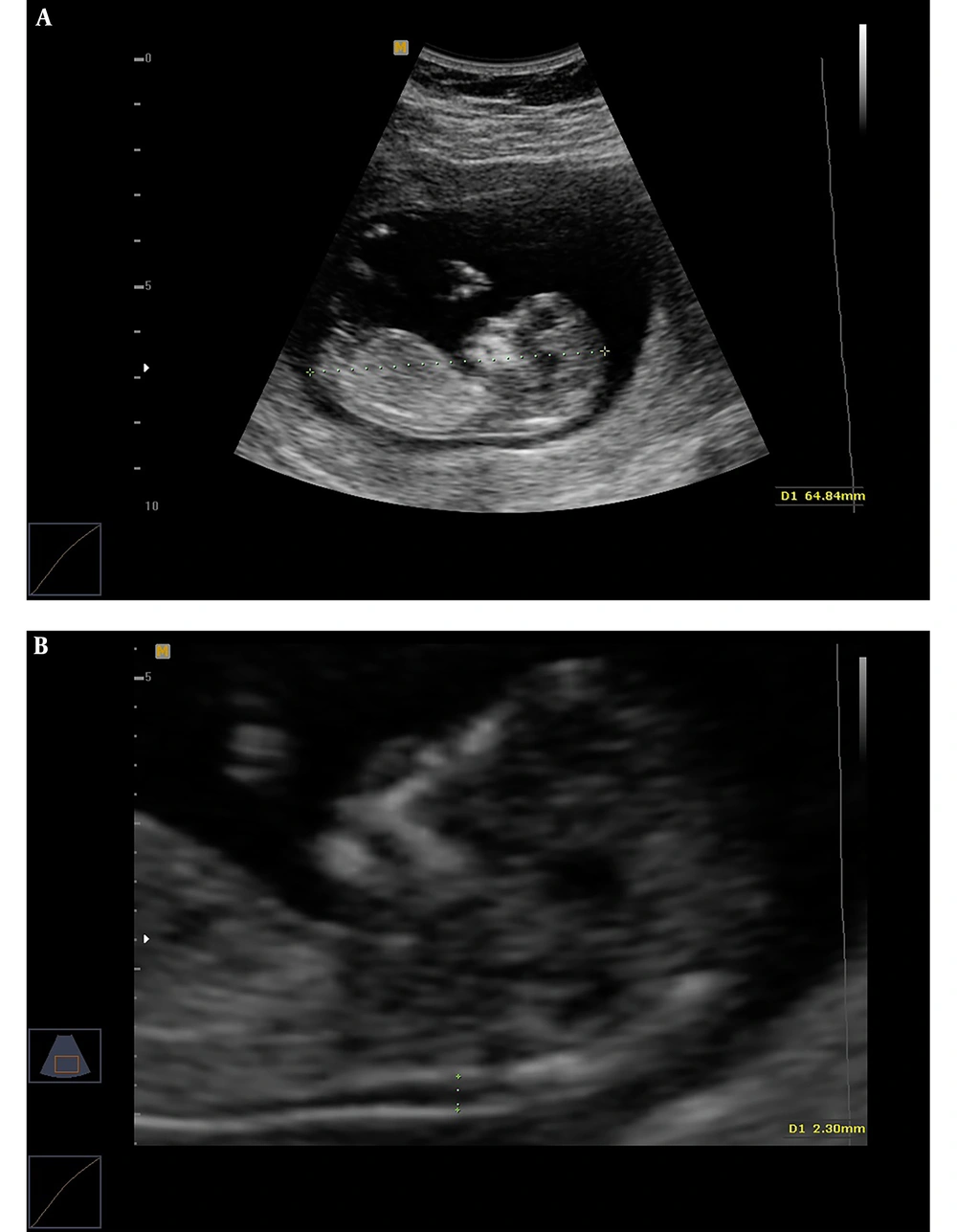
Transabdominal and, when necessary, transvaginal ultrasound were performed by an ultrasound specialist to diagnose any major fetal defects and determine fetal CRL and NT thickness. For NT assessment, the fetus should have been in a neutral position, with the head aligned with the spine. Care must be taken to distinguish between fetal skin and the amnion. The widest part of translucency was measured (Figure 1). Serum samples were collected and analyzed to determine the concentrations of free β-hCG and PAPP-A using an automated machine (Kryptor system, Brahms AG, Berlin, Germany). To convert their concentrations into Multiple of the Median (MoMs), free β-hCG and PAPP-A serum levels were divided by expected gestational age-specific medians. Ultrasonographic and biochemical results were recorded in a database provided by the FMF risk calculation software (Logical Medical Systems, London, UK). Finally, the estimated risks of DS were defined in women aged under and over 35 years old. Finally, according to the report retrieved by the software, DS risk was classified into low (over 1:1000), moderate (below 1:1000), and high (below 1:100) categories. After combined FTS, amniocentesis was performed to obtain fetal karyotype in the patients with an estimated risk of below 1:100. Women with low and moderate risks and a family history of genetic diseases underwent elective amniocentesis. All delivery outcomes were reviewed and examined by a pediatrician in order to diagnose trisomy 21 according to physical and developmental problems and the results of the karyotype test.
3.3. Statistical Analysis
The data were analyzed using SPSS 16.0 software package (SPSS Inc, Chicago, IL). Fetal CRL, NT thickness, as well as maternal PAPP-A and β-hCG serum levels were compared between different age groups using analysis of variance (ANOVA) followed by Tukey’s test. Data were expressed as mean ± SD, and the level of statistical significance was set at P < 0.05. Risk assessment and estimating DS detection rate, as well as the false positive and false negative rates were conducted according to the results of the FMF risk calculation software and presented as percentages in different age groups.
4. Results
Pregnant women under and over 35 years of age comprised, respectively, 85.41% and 14.59% of the study population (Table 1). The mean gestational age was 12.5 ± 0.7 (range: 11-13.6) weeks.
| Age Groups, y | No. (%) |
|---|---|
| 20 - 25 | 1783 (24.80) |
| 26 - 30 | 2435 (33.85) |
| 31 - 35 | 1925 (26.76) |
| 36 - 40 | 1049 (14.59) |
| < 35 | 6143 (85.41) |
| > 35 | 1049 (14.59) |
| Total | 7192 (100) |
Significant differences were observed in fetal CRL between various age groups (Table 2). The lowest mean fetal CRL (56.60 ± 11.3 mm) was observed in the age group of 20 - 25 years, and the highest mean (59.55 ± 8.3 mm) was related to the age group of 31 - 35 years. Fetal CRL showed a significant correlation (r = 0.064, P < 0.001) with maternal age. There was no significant difference (P = 0.461) in fetal CRL between women with ages under (57.81 ± 7.5) and over (57.59 ± 7.5) 35 years. There was a significant difference in the mean fetal NT thickness between various age groups (Table 2). The lowest mean fetal NT thickness (1.70 ± 0.39 mm) was observed in the age group of 20-25 years, and the highest (1.85 ± 0.41 mm) was seen in the 31-35-year age group. Fetal NT thickness showed a significant correlation (r = 0.083, P < 0.001) with maternal age. No significant difference (P = 0.086) was seen between women aged under (1.74 ± 0.41) and above (1.71 ± 0.46) 35 years.
| Age Groups, y | CRL, mm | NT, mm |
|---|---|---|
| 20 - 25A | 56.60 ± 11.3B, C, D | 1.70 ± 0.39C |
| 26 - 30B | 57.71 ± 7.8A, C | 1.68 ± 0.41C |
| 31 - 35C | 59.05 ± 8.3A, B, C | 1.85 ± 0.41A, B, C |
| 36 - 40D | 57.59 ± 7.5A, C | 1.71 ± 0.46C |
| < 35 | 57.81 ± 7.5 | 1.74 ± 0.41 |
| > 35 | 57.59 ± 7.5 | 1.71 ± 0.46 |
Abbreviations: CRL, crown-rump length; NT, nuchal translucency.
aA, B, C, and D, there is a significant difference between groups with different letters; *, there is a significant difference (P < 0.05) between pregnant women under and over 35 years of age.
Serum levels of PAPP-A and β-hCG were the lowest in pregnant women aged 36 - 40 years and the highest in those aged 26 - 30 years (Table 3). There was a significant negative correlation (r = -0.042, P < 0.001) between serum PAPP-A level and maternal age. Serum PAPP-A was also significantly (P < 0.001) different between pregnant women under (26.9 ± 13.6) and over (25.2 ± 11.7) 35 years of age. A significant negative correlation (r = -0.093, P < 0.001) was observed between serum β-hCG level and maternal age. There was a significant difference (P < 0.001) in serum β-hCG level between pregnant women under (116.5 ± 83.6) and over (95.5 ± 60.9) 35 years of age. The mean values of PAPP-A MoM and β-hCG MoM in different age groups have been shown in Table 3. Both PAPP-A MoM and β-hCG MoM showed a significant correlation (r = -0.43, P < 0.001 and r = -0.98, P < 0.001, respectively) with maternal age. Also, significant differences (P < 0.001) were seen comparing PAPP-A MoM and β-hCG MoM between pregnant women aged under and over 35 years of age.
| Age Groups, y | PAPP-A, mg/L | β-hCG, ng/mL | PAPP-A MoM | β-hCG MoM |
|---|---|---|---|---|
| 20 - 25A | 26.9 ± 13.8D | 117.7 ± 84.8C, D | 1.23 ± 0.65C, D | 1.02 ± 0.70C, D |
| 26 - 30B | 27.7 ± 14.6C, D | 123.9 ± 93.2C, D | 1.26 ± 0.65C, D | 1.06 ± 0.80C, D |
| 31 - 35C | 25.8 ± 12.1B | 106.7 ± 67.4A, B, D | 1.16 ± 0.57A, B | 0.91 ± 0.57A, B, D |
| 36 - 40D | 25.2 ± 11.7A, B | 95.5 ± 60.9A, B, C | 1.15 ± 0.55A, B | 0.83 ± 0.48A, B, C |
| < 35 | 26.9 ± 13.6* | 116.5 ± 83.6* | 1.22 ± 0.63* | 0.99 ± 0.71* |
| > 35 | 25.2 ± 11.7 | 95.5 ± 60.9 | 1.15 ± 0.55 | 0.83 ± 0.48 |
Abbreviations: β-hCG, β-human chorionic gonadotropin; PAPP-A, pregnancy-associated plasma protein-A.
aA, B, C, and D, there is a significant difference between groups with different letters; *, there is a significant difference (P < 0.05) between pregnant women under and over 35 years old.
Screening assessment based on a combination of fetal CRL, NT thickness, and maternal serum PAPP-A and β-hCG at around 11 - 13 weeks of gestation showed that 6.47% (466/7192) of the studied population were at a high risk of DS (Table 4). A significant correlation (r = 0.137, P < 0.001) was observed between maternal age and the high risk of DS based on the combined FTS strategy at around 11 - 13 weeks of gestation. Among 292 (4.06%) women with a high risk of DS, 254 underwent amniocentesis, while 38 cases declined further testing and terminated the pregnancy. Only 317 of the women with a moderate risk of DS (1006/7192) underwent amniocentesis. Moreover, among 5894 low-risk women, 135 underwent amniocentesis. Among 706 women who underwent amniocentesis, 26 abnormal karyotypes were observed (19 cases of trisomy 21, including 17 women at the high and two at the low risk groups for DS, and seven cases of trisomy 18). The total number of false positive cases was 266. Among 5894 women in the low-risk group for DS, two trisomy 21 cases were born, who failed to be detected by FTS.
| Age Groups, y | Risk (%) | Detection Rate (%) | False Positive Rate (%) | False Negative Rate (%) | ||
|---|---|---|---|---|---|---|
| Low | Moderate | High | ||||
| 20 - 25 | 93.4 (1666) | 3.2 (57) | 3.4 (60) | 75 (3) | 3.1 (57) | 0.05 (1) |
| 26 - 30 | 84.3 (2052) | 14 (343) | 1.7 (40) | 80 (4) | 1.4 (36) | 0.04 (1) |
| 31 - 35 | 81.1 (1562) | 16.6 (320) | 2.3 (43) | 100 (3) | 2.07 (40) | 0.0 (0) |
| 36 - 40 | 58.5 (614) | 27.3 (286) | 14.2 (149) | 100 (7) | 13.5 (142) | 0.0 (0) |
| < 35 | 85.9 (5280) | 11.7 (720) | 2.4 (143) | 83.3 (10) | 2.1 (133) | 0.03 (2) |
| > 35 | 58.5 (614) | 27.3 (286) | 14.2 (149) | 100 (7) | 13.5 (142) | 0.0 (0) |
5. Discussion
The risk of a baby being born with anomalies and structural defects is 3% to 5% for each pregnancy (9). At the forefront of these disorders is DS, a genetic disease that is often non-inherited. Although having a child with DS is directly related to the mother’s age, it has been reported that more than 60% of children with trisomy 21 are born to pregnant women aged under 35 years. Therefore, the early diagnosis of DS should not be only focused on pregnant women over 35 years of age, and screening tests in the first trimester of pregnancy are essential for mothers younger than 35 years of age as well (6, 10). The present study evaluated the performance of FTS in assessing the risk of trisomy 21 during the first trimester of pregnancy in women under and over 35 years of age in Southwest Iran. The overall detection and false positive rates of FTS were calculated using a 1:100 risk cut-off value. In the present study, among 7192 cases screened, there were a total of 5894 cases with FTS risk scores greater than 1:1000, 1006 cases with FTS risk scores between 1:100 and 1:1000, and 292 cases with FTS risk scores lower than 1:100.
Out of 706 pregnant women undergoing amniocentesis (as an invasive diagnostic test), abnormal karyotypes were returned in 26 cases, and the total number of DS false positive cases was 275. So, using a combination of fetal CRL and NT thickness, as well as maternal serum free β-hCG and PAPP-A levels, the overall detection rate of FTS for DS was obtained 89.47%. The false positive rate of FTS for DS was 3.82% that falls into the often-quoted range of 3.5% - 5.0% (5). The total number of false negative cases was two (0.028%) with FTS risk scores greater than 1:1000. The detection rate of FTS for DS alone was found to be lower in women under 35 years of age than over 35 years (83.3% vs. 100%, respectively). There was also a lower false-positive rate for detecting DS in women under 35 years of age compared with women aged > 35 years (2.1% vs. 13.5%, respectively). It could be concluded that the performance of FTS is nearly similar in young and older women (i.e., over 35 years). The DS detection rate observed in the present study is comparable to the rates reported in other populations. According to the guidelines of the Society of Obstetricians and Gynecologists of Canada, the detection rate of DS during the first trimester was 83%, and the false positive rate was 5%, which were in line with the findings of the present study (11). In a similar study conducted during 2010 and 2013 by Seyyed Kavoosi et al. (12) on 25783 pregnant women, they found that the detection rate of FTS for DS in Tehran (Iran’s Capital) was 84.2%, and the false positive rate was 5.17%. Benn (13) also reported the detection rates of 80% and 93% and the false-positive rates of 2.8% and 13.2% in women under and over 35 years of age, respectively. Avgidou et al. (14) showed that in women younger and older than 35 years of age, the detection rates were 95.6% and 95.3%, and the false-positive rates were 12.57% and 10.43%, respectively. Moreover, Yang et al. (15) found that in women aged < 35 and > 35 years, the detection rates were 68.8% and 71.4%, and the false-positive rates were 4.3% and 15.9%, respectively. In another study conducted by Wright et al. (16), the detection rates were 83.71% and 91.85%, and the false-positive rates were 1.5% and 6.1% in women aged < 35 and > 35 years, respectively. Furthermore, Li et al. (17) reported that the false-positive rates were, respectively, 5.43% and 1.33% in women under and over 35 years of age, respectively. Li et al. (17) also found a similar efficacy for FTS in women aged under and over 35 years (87.5% vs. 85.7%, respectively). However, in a study conducted by Peuhkurinen et al. (6), a significantly poorer detection rate was reported in young women (74.0%) compared to older women (87.0%), which could be due to using the Perkin Elmer Lifecycle TM algorithm instead of the FMF software for analyzing screening results.
Furthermore, this study showed that there was a significant correlation between the risk of DS and maternal age. The risk ratio of DS (1:100) was found to be higher in women over 35 years of age than in those aged under 35 years (14.2% vs. 2.4%, respectively). There was also a higher DS moderate risk (1:100 - 1:1000) in women aged over 35 years compared with women under 35 years of age (27.3% vs. 11.7%, respectively). Our data also showed that the prevalence of DS was 2.64 per 1000 births in Ahvaz, Southwest Iran. The highest prevalence of DS was found in pregnant women aged 36 to 40 years. In addition, the prevalence of DS was higher in pregnant women aged 35 years compared with those aged under 35 years (6.67 vs. 1.95 per 1000 cases, respectively). Liu et al. (18) found that the prevalence of DS in Taiwan was 0.4% and 1.3% in pregnant women under 35 years and older, respectively, during 1999 and 2002. Siffel et al. (19) reported that between 1990 and 1993, the prevalence of DS in Atlanta was 24.7 per 10000 births in women over 35 years of age compared to 6.8 per 10000 in women under 35 years of age. According to Seyyed Kavoosi et al. (12), the prevalence of DS was related to maternal age, reporting a DS prevalence of 1 per 438 cases in Tehran, Iran, during 2010 and 2013. In a study conducted by Li et al. (17) from 2006 to 2011, they showed that the prevalence of DS in Singapore was 3.6 per 1000 cases. Glivetic et al. (20) also reported that the total prevalence of DS (2009 - 2012) in Croatia was 7.01 per 10000 births. Jaruratanasirikul et al. (21) found that during 2009 - 2013, the prevalence of DS in Southern Thailand significantly increased with maternal age, with a significantly higher incidence in older women. They reported that the prevalence of DS per 1000 births was 0.47 in mothers younger than 30 years, 0.88 in mothers between 30 and 35 years, and 4.74 in mothers above 35 years (21). In this regard, Elahifar et al. (22) demonstrated that 57% of infants with DS were born to mothers older than 35 years (23). However, Yang et al. (15) concluded that the risk of DS was not solely related to maternal age, reporting a notable incidence of DS in women younger than 35 years.
One of the limitations of our study was that we did not obtain karyotypes from the women who declined further testing and terminated their pregnancies. In other words, the prevalence of DS might have been underestimated in the present study. Moreover, since people with different ethnicities live in Southwest Iran, further studies are needed to divulge the possible role of other parameters in determining the efficiency of DS screening.
5.1. Conclusions
This study provided first estimates on the maternal age-specific prevalence of trisomy 21 in women of different ages in Ahvaz, Southwest Iran. Our data showed that the prevalence of DS was 2.64 per 1000 births in this region. The prevalence of DS was 1.95 vs. 6.67 per 1000 cases in pregnant women under 35 years of age and older, respectively. Our findings also demonstrated that FTS was an effective method of screening for trisomy 21 in women under 35 years of age and older. Accordingly, FTS delivered the overall detection rate of 89.47% and the false-positive rate of 3.82% for DS. The present study showed a positive correlation between maternal age and the prevalence of DS. However, regardless of maternal age, clinicians should recommend FTS as a first-line screening method for DS and provide appropriate training programs about these screening methods for early DS diagnosis.