1. Background
Staphylococcus aureus is a major encountered Gram-positive organism that causes a wide variety of infections in both the community and hospital (1). This important medical bacterium is a leading cause of diverse clinical infections ranging from mild and straightforward infections to severe and life-threatening diseases (1-3). This notorious pathogen, especially multidrug-resistance strains, in particular methicillin-resistant Staphylococcus aureus (MRSA), can infect approximately every site of the human body (4). Nowadays, MRSA emerged as one of the problematic nosocomial pathogens globally (2). It has been reported that infection caused by MRSA can increase morbidity, risk of mortality, prolonged hospital length of stay, and financial costs (5). Although MRSA can colonize several body sites, anterior nares are the most common colonization niche for this organism (5, 6). Healthcare workers (HCWs) and patients can harbor MRSA in the nasal cavity transiently or be persistent colonizers (2, 7). Nasal carriers, especially HCWs, can serve as reservoirs and sources for transmission of MRSA to hospitalized patients (7, 8).
Furthermore, the transmission of MRSA from carrier individuals could happen via patients' contact or aerosolization following sneezing (8). Furthermore, HCWs carriers, who are at the interface between community and hospital, may be responsible for disseminating and increasing MRSA in the community (8, 9). Knowledge of the carriage rate among hospital staff and patients is necessary to control and prevent MRSA infections (6, 7).
2. Objectives
The aim of this study was to determine the frequency of MRSA nasal carriage, and its antibiotic resistance pattern among staff and patients admitted to Shahid Mohammadi Hospital in Bandar Abbas, South of Iran.
3. Methods
This cross-sectional study was conducted in Shahid Mohammadi, a teaching hospital affiliated to Hormozgan University of Medical Sciences (HUMS), Bandar Abbas, a city located in the south of Iran. During the study period from November 2017 to December 2018, 400 nasal swabs were taken from hospital staff and patients who were hospitalized.
3.1. Specimen Collection
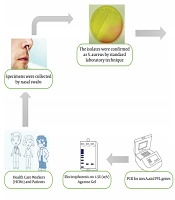
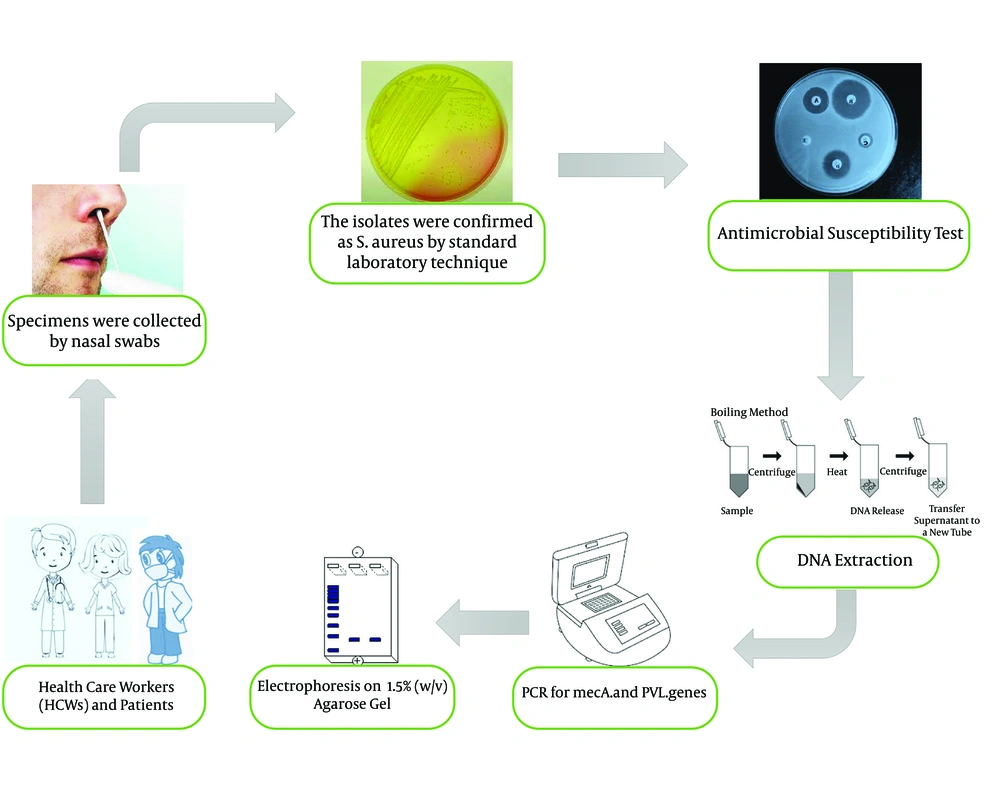
Specimens were collected with two pre-moistened cotton swabs with saline (one swab for each nostril). The samples were obtained carefully by inserting a sterile swab 2 - 3 cm into each nostril and rotating gently for five times both clockwise and counterclockwise. Swabs were inoculated on a Mannitol Salt Agar plate (Merck, Germany) and incubated at 37°C overnight (7). Mannitol fermented colonies (golden yellowish) presumptively were considered S. aureus and re-streaked on nutrient agar (Merck, Germany) and incubated at 37°C for 24 hours. The isolates were confirmed as S. aureus by Gram stain, catalase, tube coagulase, and DNase test (2, 9).
3.2. Antimicrobial Susceptibility Test
All S. aureus isolates were tested by disc diffusion test on Mueller-Hinton agar plate (Merck- Germany) according to the Clinical and Laboratory Standards Institute (CLSI) recommendations as updated in 2019 (10). For each isolate, using the direct colony suspension method, 3 to 5 colonies of fresh S. aureus culture were suspended in saline to obtain a turbidity equivalent to a 0.5 McFarland standard (10). Furthermore, bacterial suspension was inoculated on a sterile Mueller-Hinton agar plate using a cotton swab. The following commercially discs (MAST Group Ltd, Merseyside, UK) were tested: Clindamycin 2 µg, Erythromycin 15 µg, Rifampin 5 µg, Trimethoprim-sulfamethoxazole 1.25/23.75 µg, Tetracycline 30 µg, and Linezolid 30 µg. D-Zone test was carried out according to CLSI recommendations. Methicillin-resistant isolates were identified using 30 µg Cefoxitin disc (10). According to the CLSI guideline, S. aureus ATCC 25923 was used as a reference strain for quality control throughout the study (10).
3.3. Minimum Inhibitory Concentration of Vancomycin and Mupirocin
Agar dilution was carried out to determine vancomycin and mupirocin minimum inhibitory concentration (MIC). A stock solution of each mupirocin and vancomycin (Sigma Aldrich) was prepared separately, and after filter sterilization was added to molten Mueller-Hinton agar (Merck- Germany) at concentrations of (0.25, 1, 2, 4, 8, 16, 32, 64, 128, and 512 µg/µL) and poured to sterile perti dish (11). Results for vancomycin was interpreted according to CLSI guideline (10). Mupirocin breakpoint was considered according to Lee et al., as susceptible ≤ 4 µg/mL; low-level resistant, 8 - 256 µg/mL; and high-level resistant, ≥ 512 µg/mL (12). Staphylococcus aureus ATCC 29213 was used as quality control.
3.4. DNA Extraction
Genomic DNA of S. aureus was extracted with the boiling method with a minor modification. Briefly, S. aureus isolates were grown on Mueller-Hinton agar plate overnight. Next, five colonies of bacteria were picked for each isolate and dissolved in 100 µL of TE buffer (10 mM Tris, 1 mM EDTA, pH 7.8). The microtubes were incubated in the dry block (BOECO, Germany) for 10 min at 100°C and immediately placed on ice after this time, followed by centrifugation at 9,000 g at 4°C for 30 seconds. The supernatant was transferred to a new 0.5 µL microtube and used as a DNA template (13).
3.5. PCR for mecA and PVL Genes
All isolates were tested for mecA and PVL with specific primers mecA1 (5'-GTAGAAATGACTGAACGTCCGATAA-3') mecA2 (5'-CAATTCCACATTGT TTCGGTCTAA-3') and Luk-PV-1 (5'-ATCATTAGGTAAAATGTCTGGACATGATCCA-3') and Luk-PV-2 (5'-GCATCAAGTGTATTGGATAGCAAAAGC-3') (14). The amplification reaction was performed in a final volume of 25 µL containing 1X PCR buffer, 1U Taq polymerase, 2mM MgCl2, 200µM of dNTP (SinaClon, Bioscience Co, Iran), 0.4 µM of each primer (TAG, Copenhagen A/S Denmark) and 2 µL of extracted DNA. PCR condition was programmed in DNA thermal cycler (Bio-Rad My Cycler Thermal Cycler) with the following condition: Initial denaturation 94°C for 5 min then 30 cycles of denaturation at 94°C for 45 s, annealing at 55°C for 45 s, extension at 72°C for 1 min and a final extension at 72°C for 5 min. The PCR products were separated by electrophoresis on 1.5% (w/v) agarose gel (SinaClon, Bioscience Co, Iran) in 1X TBE buffer, stained by 5X GelRed (Biotium, USA), and visualized on a gel documentation system (13). Staphylococcus aureus ATCC 33591 was used as a positive control for mecA gene, and a clinical isolates S. aureus containing PVL with Accession number: HG937618 was used as PVL positive control. The process of methods is summarized in Figure 1.
3.6. Statistical Analysis
The frequency analysis, standard deviation (SD), means, and percentages were calculated using the statistical program for social sciences (SPSS) version 22 (SPSS version 22) (IBM, Chicago, IL, USA).
3.7. Ethical Consideration
This study was approved by the Ethics Committee of Hormozgan University of Medical Sciences (HUMS.REC.1396.67).
4. Results
4.1. Clinical Isolates
Four hundred participants (130 HCWs and 270 patients) were enrolled in this study from November 2017 to December 2018. Overall, 32 S.aureus isolates were obtained, and 8% of the participants were nasal carriers. Of 130 HCWs screened, 11 (8.5%) subjects were nasal carriers of S. aureus, whereas, of 270 patients, 21 (7.8%) patients were positive for S. aureus.
4.2. Antimicrobial Susceptibility Test
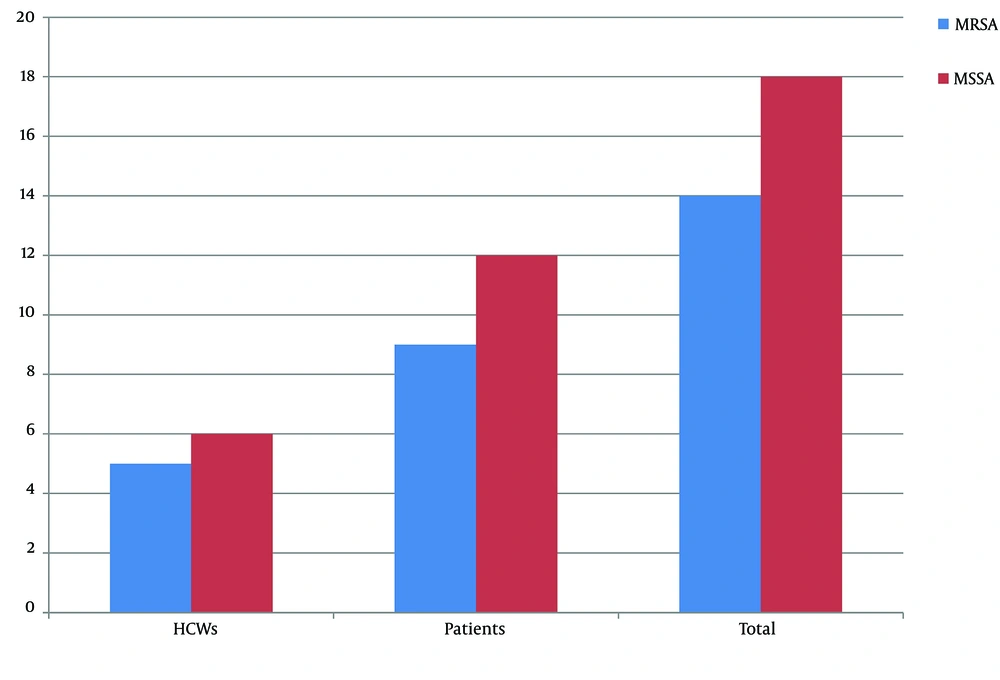
The results of disc diffusion are shown in Table 1. Accordingly, the most effective antibiotic against S. aureus isolates in vitro was linezolid, and 100% of the isolates were susceptible to this drug. A high resistance rate was observed for erythromycin, and 46.9% of isolates were resistant to this antimicrobial agent and showed the lowest effect on S. aureus isolates. Of 32 isolates, 14 isolates (43.8%) were methicillin-resistant, and 18 isolates (56.3%) were methicillin-sensitive. Furthermore, of the 11 isolates collected from HCWs, 5 (45.5%) isolates were MRSA, and 6 (54.5%) isolates were methicillin-susceptible. On the other hand, 9 (42.9%) and 12 (57.1%) S.aureus isolates collected from the patients were MRSA and MSSA, respectively (Figure 2). Moreover, D-Zone positive was observed in 4 (12.5%) isolates, and these isolates showed inducible clindamycin resistance, whereas the remaining 28 (87.5%) isolates were negative in D-test.
| Antibiotics | Sensitive | Intermediate | Resistant |
|---|---|---|---|
| Linezolid | 32 (100) | - | - |
| Rifampin | 28 (87.5) | - | 4 (12.5) |
| Trimethoprim-sulfamethoxazole | 26 (81.3) | - | 6 (18.8) |
| Gentamicin | 25 (78.1) | 1 (3.1) | 6 (18.8) |
| Tetracycline | 21 (65.6) | - | 11 (34.4) |
| Clindamycin | 19 (59.4) | - | 13 (40.6) |
| Erythromycin | 17 (53.1) | - | 15 (46.9) |
The Results of Antibiotics Resistance Profile Among Staphylococcus aureus Isolates a
4.3. MIC Results
Agar dilution results for mupirocin showed that 20 (62.5%) isolates had MIC ≤ 4 µg/ml and was considered sensitive, 10 (31.3%) isolates had low-level resistant MIC = 16 µg/ml, and 2 (6.3%) isolates had MIC = 512 and were categorized as high-level mupirocin-resistant. Furthermore, for vancomycin, all isolates had MIC ≤ 2 and were susceptible to vancomycin.
4.4. PCR Results for mecA and PVL Genes
Fourteen (43.8) isolates were mecA-positive and recorded as methicillin-resistant, and 18 (56.3%) were mecA-negative and methicillin-sensitive. All isolates were PVL-negative.
5. Discussion
The S. aureus nasal carriers among HCWs are a major concern because they serve as a potential reservoir for spreading this organism to critically ill patients and susceptible individuals (5, 15). In this study, we assessed the prevalence of S. aureus nasal carriage in HCWs and patients who were hospitalized at Shahid Mohammadi Hospital in Bandar Abbas, South of Iran. Our results revealed that 8.5% of the HCWs and 7.8% of the patients were nasal carriers. In Iran, according to a systematic review from 2000 to 2016, the average nasal carriage rate among HCWs has been reported 22.7% (19.3 - 26.6%) (5). However, in two newer studies from Iran, the frequency of nasal carriage was 30.16% and 10.8%, respectively (16, 17). To date, the universal carriage rate among HCWs is unclear, and various frequencies have been reported from 12% to 48% in different countries (2, 3, 7-9, 18). The rate of nasal carriage in our study is lower than in other reports. This is likely due to effective infection control programs in the studied hospital.
Furthermore, MRSA rate in our study is 45.5% and 42.9% in HCWs and patients, respectively. In Iran, MRSA rate in HCWs is 26% to 46.7% (5, 16, 17), and our finding is consistent with the previous studies. The distribution of MRSA among nasal carriers varies from 5.7 to 82.3% in different regions of the world, and our results are similar to the mentioned studies (2, 3, 6-9). In addition, the variation of nasal carriage rates in different parts of the world may be due to sample size, study period, microbiological techniques, infection control strategies of each hospital, and staff awareness about MRSA (2, 5, 8). In our study, the antimicrobial susceptibility test results demonstrate that vancomycin and linezolid were the most effective antibiotics against S. aureus isolates. Although vancomycin-resistant S. aureus has previously been observed in different regions (19), surprisingly, all of our isolates were susceptible to vancomycin, and this drug can be used in the treatment of these isolates. Although linezolid resistance has been reported (20, 21), we could not find any resistant isolates, and all of our isolates were sensitive. Linezolid, the first approved oxazolidinone by the US Food and Drug Administration (FDA), is an important alternative option to vancomycin in the treatment of MRSA infections (20, 22). Since HCWs are at high contact with patients, regular screening of healthcare workers and appropriate infection control measures and decolonization have an important role in controlling MRSA transmission within hospitals (2, 5, 15, 23, 24). Mupirocin is widely used for the decolonization of MRSA and MSSA in healthcare personnel as well as patients. Owing to the widespread use of this valuable drug, the mupirocin-resistant isolates have been emerged (12, 23). According to a systematic review, the overall prevalence of high-level mupirocin-resistant S. aureus (HLMuRSA) is 7.6% (23). The rate of HLMuRSA in our isolates was 6.3% that is near to reported global range. Interestingly, 62.5% of the isolates were susceptible to mupirocin, and it seems this antibiotic yet can be used for decolonization.
5.1. Conclusions
The findings of the present study demonstrate that the S. aureus carriage rate among personnel and patients is relatively lower than other studies, but the frequency of MRSA is comparable with previously reported ranges and is approximately high among HCWs and patients. Linezolid and vancomycin are the most potent antimicrobial agents and can be used for the treatment of S. aureus infections in Shahid Mohammadi Hospital. Since the MRSA carriage rate is high, an effective infection control strategy and appropriate decolonization program should be adopted to prevent the transmission of such isolates.
5.2. Limitations of the Study
In the present study, we cannot detect persistent or transient carriage among HCWs, and further studies are needed to clarify this situation. Another limitation was that we could not define the carriage from each hospital ward separately.