1. Background
Autophagy is the natural, regulated mechanism of the cell that removes unnecessary or dysfunctional components (1). The intracellular mechanism of autophagy is activated with age and entering old age, which is associated with a decrease in the size muscle fibers, the number of muscle fibers, and the production of contractile proteins (2).
Autophagy function impairment may also impair the function of some specific cells and tissues (3). In this regard, non-alcoholic fatty liver disease is caused by successive storage of fat in liver cells, followed by impaired insulin secretion and insulin sensitivity (4).
Approximately, 30 genes regulate the autophagy process (5), among which Beclin-1 and LC3 play a greater role in autophagy (6). Changes in mtor, Becline-1, and p62 central autophagy genes play a very important role in sarcopenia due to type 2 diabetes mellitus (T2DM) (7, 8).
Numerous studies have suggested that autophagy is very important for cell protection and survival (9). But according to reports, physical activity can affect autophagy regulatory genes (10). Resistance training is probably more beneficial by creating muscle hypertrophy and improving glycemic profile (11). Physical exercise promotes muscle adaptation, which affects autophagy system as well as the status of diabetes and fatty liver (12). Some studies suggest that long-term strength can cause oxidative stress in most of the body's peripheral tissues.
The function of the autophagy system lysosomes are important in the destruction of proteins, and intracellular organs of skeletal muscle (13). By increasing age, changes in body composition occur that include metabolic diseases, biological changes, and reduced balance in the performance (14). The results of Madsen (2015) showed that short-term exercise can be a good alternative for positive changes in risk markers of T2DM (15). Ha et al. (2015) showed that exercise on plasma malondialdehyde concentration and superoxide dismutase activity causes a significant change in oxidative stress (16). There is evidence to suggest that insulin resistance may increase with increasing oxidative stress and that antioxidant therapy may be effective (17).
To counteract an inflammatory response, the supplementation of substances and antioxidant capabilities is practiced after physical exercise. One of these substances is alpha-lipoic acid (ALA) (18), which is a well-known supplement for preventing or delaying oxidative stress and inflammation. Intense resistance training has been proposed as an effective treatment in T2DM, and the most important factor in the volume of exercise in metabolic control and cardiac function (19, 20).
Damage to the cell membrane causes an increase in malondialdehyde (21), and cell dysfunction (22). Researchers have reported that moderate-intensity exercise in male rats improves muscle atrophy and inhibits the autophagy system (23). Scientists have concluded that exercise causes changes in the parameters of autophagy and diabetes mellitus with fatty liver (3).
2. Objectives
Several studies have shown that methods of training and supplements are effective in progressive effects of autophagy. However, the results are different. Therefore, this study aimed to investigate the effects of resistance training with ALA on Becline-1 gene expression and malondialdehyde serum concentrations in gastrocnemius and soleus muscle tissue's elderly rats with T2DM with fatty liver.
3. Methods
This research is experimental and post-test design in five groups.
3.1. Rats
The statistical population was 100 old male Wistar rats that had not been studied until the implementation of the training protocol. Rats were purchased from the Pasteur Institute and transferred to the animal room of the university laboratory. Among them, 35 rats were randomly selected as subjects. In this study, rats were kept in separate polycarbonate cages measuring 27 20 × 27 × 47 cm. Ambient temperature was set at 22 ± 1.4°C, the light cycle was set at 12:12 pm, and humidity was set at 55% ± 0.6. All stages of the research were carried out in accordance with the ethical principles of working with animals. After a week of familiarity with the laboratory environment, the rats, first became diabetic, and then their liver became oily and were randomly divided into five groups, including healthy control, diabetic, diabetic + resistance training, diabetic + supplement, diabetic + resistance training + supplement.
3.2. Exercise Protocol
The resistance training protocol included 15 times climbing a special ladder to a height of 1 meter and 46 steps with a distance of 2 cm. A weight was attached to the tails of the rats. In case of refusal to climb, low watt electric shock was used to stimulate the animal. After two weeks, the rats performed resistance training for 10 weeks, including five sessions per week and each session for 40 minutes (24).
3.3. Make Diabetes and Fatty Liver
For inducing T2DM in the study sample, after 12 hours of fasting, a solution of nicotine amide dissolved in normal saline at a dose of 120 mg/kg. After 15 minutes, streptozotocin was used 0.1 M citrate buffer solution was injected intraperitoneally at a dose of 65 mg/kg. By examining blood samples of the eye and fasting glucose above 426 mg/dL, we confirmed that the rats were diabetic (25). To create fatty liver, the healthy control group was fed a standard diet of rodents, and the other groups were fed a high-fat diet for 10 weeks (26). Serum cholesterol concentration was measured as one of the indicators of the fatty liver at the end of the study. ALA supplement, at a dose of 50 mg/kg as a solution of dimethyl sulfoxide, three times a week by intraperitoneal injection was given to rats in the training + groups used. Rats in the training group, the healthy control group, and the patient control group received 5% normal saline solution.
3.4. Tissue Sampling and Laboratory Measurements
Forty-eight hours after the last training session, the subjects underwent intraperitoneal injection of a combination of ketamine and xylazine anesthesia and tissue and blood samples. Gastrocnemius and soleus muscle tissues were isolated and stored at -80°C, and blood samples were taken from a vein after surgery, and then sent to the laboratory. Malondialdehyde serum level was determined by the laboratory TBA method. The expression of Becline-1 gene was evaluated in gastrocnemius and soleus muscle by RT-PCR (27).
3.5. Statistical Analysis
The normality of the data was assessed using the Shapiro-Wilk test, and the homogeneity of variances was assessed by the Leven test. One-way analysis of variance was used to examine the differences between groups. Tukey post hoc analysis for multiple comparisons was used to determine between-group differences (P < 0.05, SPSS statistical software, version 21).
4. Results
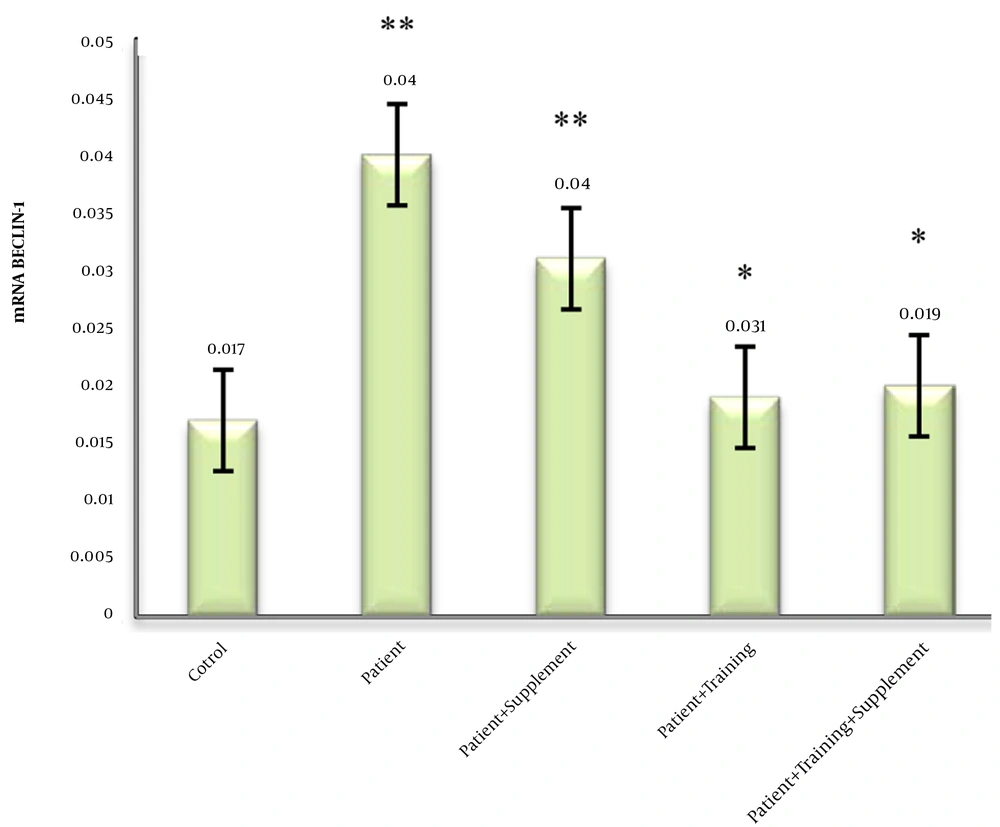
4.1. Effects of ALA and Resistance Training on Becline-1
The mean, standard deviation, and percentage changes of Becline-1 in different study groups were calculated. One-way analysis of variance showed significant differences between groups (P < 0.05). Tukey post hoc test showed a significant increase in the patient group compared to the healthy group (P = 0.001). The difference between the patient groups was not significant (P = 0.999). On the other hand, a significant decrease was observed in the groups of patient + supplement (P = 0.001), patient + resistance training (P = 0.001), patient + resistance training + supplement (P = 0.025) compared to the patient group (P = 0.001). Data analysis showed that there was no significant difference between the groups (Figure 1). A period of strength training and ALA supplementation alone or in combination with reduced Becline-1 gene expression in elderly diabetic rats with fatty liver.
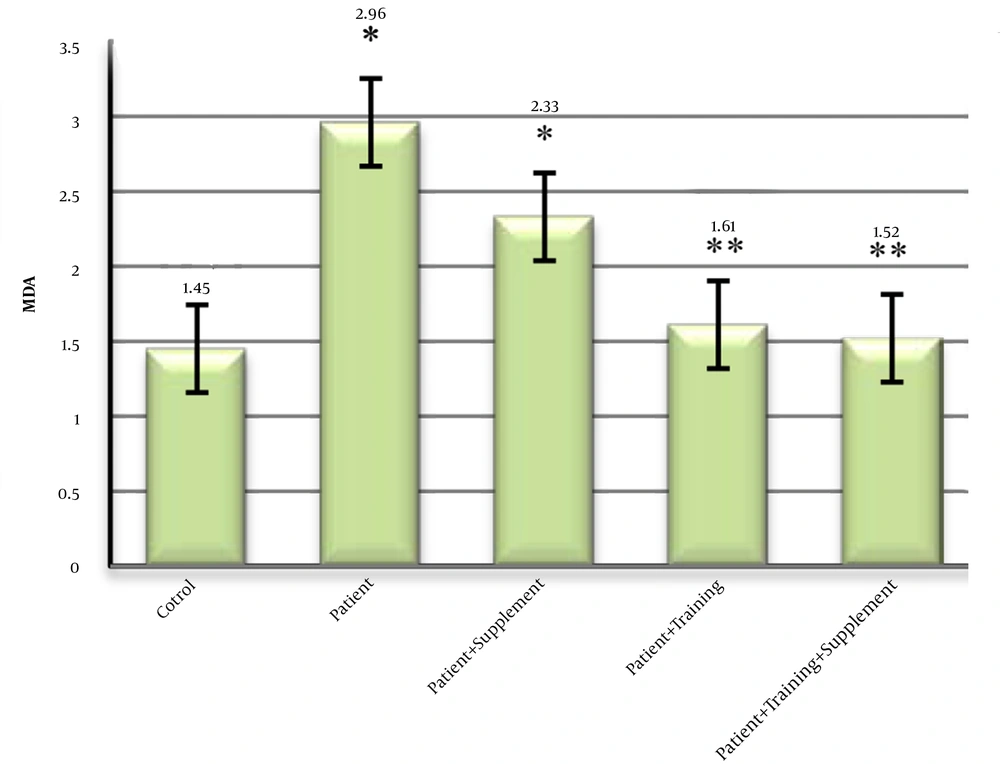
4.2. Effects of ALA and Resistance Training on Malondialdehyde
The mean concentration of malondialdehyde in diabetic rats with fatty liver had a significant increase in the patient group compared to other groups. While the control group had the lowest concentration of malondialdehyde compared to other groups. There was no significant difference in malondialdehyde concentration between the two groups (Figure 2).
5. Discussion
5.1. Response Becline-1 to ALA and Resistance Training
Autophagy is a highly regulated process for the destruction of damaged proteins and intracellular components. Becline-1 is a very important regulatory molecule in the initiation and formation of autophagosomes (28). There is very limited information about Beclin-1 following training protocol. Cho et al. (2017) studied the effect of endurance training on skeletal muscle autophagy, but there was no significant change in Becline-1 protein in soleus muscle (29). Mejias-Pena et al. (2017) studied the effect of resistance training on Becline-1 changes in elderly men and women. Their results reported a significant increase in the exercise group (3). In another study, resistance training increased Becline-1 significantly as an autophagy-regulating protein (30). Brandt et al. (2018) also examined the acute effect of aerobic exercise on Becline-1 changes in trained men. Exercise increased Becline-1 by 1.3 times compared to before exercise. But no significant change was observed in the speed training group (31). Another researcher reported a significant increase in Becline-1 following eight weeks of strength training. These results were contrary to the results of the present study. In their research, they used an intensity of 60% of oxygen consumption (31). The difference between the results and the present study is due to the difference in the method, training protocol, supplementation, and subjects.
Becline-1 is a regulatory and key gene in the autophagy process. Genetic alterations in Becline-1 in rats led to dynamic changes in autophagy (32). Beclin-1 appears to be regulated by a range of ROS activators and inhibitors, advanced glycation end products, TGF-β, and NF-κB, which have an increasing trend in diabetes (33). In general, AMPK is an intracellular energy sensor that responds to energy constraints. AMPK appears to lead to the cleavage of Beclin-1 by Bcl2, which protects against apoptosis and autophagy. It is possible, Bcl2 signaling activity and cleavage of Becline-1/Bcl2 complex can be an essential mechanism for AMPK in regulating the changes between autophagy and apoptosis pathways in diabetic conditions (34). The results of the present study showed that strength training with or without ALA supplementation is equally effective in reducing Becline-1. However, the changes were significant compared to the diabetic group with fatty liver. In other words, it indicates the anti-autophagic role of strength training. It seems that the reason for the disparity was due to healthy species versus diseased species in the present study. Becline-1 changes in healthy species after exercise are used for physiological autophagy. Because exercise as external stress causes damage to muscle fibers. So, the rate of autophagy is high in patients, and exercise plays an important role in preventing non-physiological conditions. Mejias-Pena et al. (2017) reported that Becline-1 levels increased in the resistance training group. They hypothesized that regular exercise had a preventive effect on the loss of autophagy efficiency in old age (3). In general, the results of the present study showed, strength training with ALA supplementation significantly reduces Becline-1 in diabetic rats with fatty liver. As mentioned, Becline-1 regulates autophagy in diabetics.
5.2. Response of Malondialdehyde to ALA and Resistance Training
In this study, ALA significantly decreased serum malondialdehyde level. Similar to our results, other researchers reported a decrease in oxidative stress in animals’ model of non-alcoholic fatty liver disease induced by methionine-choline-deficient diet. So, another study also showed that ALA had an effect on malondialdehyde levels in old rats. However, some studies have not reached such findings. The results of the study showed that diabetes is associated with a significant increase in hepatic malondialdehyde levels, which is in line with some previous studies (3). Oyenihi et al. (2015) observed a significant increase in malondialdehyde concentration as well as reactive oxygen species levels in the liver of diabetic rats with fatty liver. While the activity of Catalase and Superoxide dismutase enzymes were significantly reduced (35). The possible factors that may lead to elevated lipid peroxidation include an increase in free radicals generation and a decrease in enzymatic and non-enzymatic defense systems (20). In the study by Riahi et al. (2016), ALA decreased acetaminophen-induced hepatotoxicity by decreasing malondialdehyde, inducible nitric oxide synthase activity, and increasing the content of Catalase and Superoxide dismutase, and reduced Glutathione (36). These results are in line with our research findings. The effectiveness of ALA in the body on increasing antioxidant defense and inhibiting the damage caused by oxidative stress, and lowering blood sugar in diabetic patients (37). Therefore, resistance training with ALA supplementation reduced autophagy and inflammatory markers. The discrepancy observed between the results of animal studies might be attributed to the different physiological mechanisms between animals and humans in ALA metabolism and redox system regulation.
5.3. Conclusions
Strength training with ALA supplementation decreased the expression of Becline-1 and the malondialdehyde. Therefore, ALA supplementation is one of the most important factors in reducing cellular autophagy, and according to research results, strength training is another way to reduce autophagy.