1. Background
The sperm chromatin compaction occurs during the final post-meiotic phases of spermatogenesis, which plays an important role in protecting the male genome and is required for normal male fertility (1). Potential damage to development of normal embryo by sperm DNA fragmentation has emerged, which is associated with poor fertilization in vivo and in vitro, recurrent pregnancy loss, and anomalies and cancer in the offspring (2, 3). Higher levels of sperm with damaged DNA have been shown in infertile men (4). During the DNA packaging process, majority of histones are replaced firstly by transition proteins and then by protamine. Histone changes during spermatogenesis are related to the sperm protamine contents. Protamine-1 (PRM1) and protamine-2 (PRM2) are the abundant basic nuclear sperm proteins involved in condensation of the nucleus and in the integrity stability and repair of DNA. There is evidence that an altered amount of protamine may result in an incomplete chromatin condensation, and in turn, an increased susceptibility to sperm DNA damage (5). Relatively equal levels (1: 1 ratio) of PRM1 and PRM2 are expressed in fertile individuals (6). An altered amount of protamine has been shown in infertile and subfertile men that is associated with reduced embryo quality (7). A significant correlation was found between changes in protamine protein ratio and protamine mRNA ratio in sperm cells (6). Alterations in mRNA content of PRM1 and PRM2 are associated with male infertility, and abnormal PRM1: PRM2 mRNA ratios have been reported in subfertile men (8).
2. Objectives
The present study aimed to investigate DNA integrity and protamine transcripts contents in ejaculated spermatozoa of Iranian men with unexplained infertility.
3. Methods
3.1. Subjects
The Ethics Committee of the Department of Biology, Shahid Chamran University of Ahvaz, Iran, approved this study (No. 20.6.505). Written informed consent was obtained from all individuals enrolled in the study. Infertile men (n = 17) were selected from infertile couples referred to the Infertility Clinic of Imam Khomeini Hospital in Ahvaz. Healthy fertile volunteers (n = 10), whose wives achieved a pregnancy within the last year, were included as controls.
The inclusion criteria were normozoospermic men from infertile couples after 12 months of having regular unprotected intercourse whose wives had a normal reproductive function, and individuals with normal semen parameters [World Health Organization (WHO), 2010] who fathered a child during the last year. Women with a diagnosis of unexplained infertility showed normal ovulation by regular cycles and normal hysterosalpingogram. Alcohol consumers, smokers, drug addicts, and men with history of reproductive disorders and systemic diseases were excluded from the study.
3.2. Semen Analysis
After three days of sexual abstinence, semen samples were obtained by masturbation into sterile containers and allowed liquefying for 30 minutes. Sperm count, motility, and morphology were examined according to the WHO guidelines (2010). Reference values of sperm parameters based on WHO (2010) criteria were used for the interpretation of semen analysis (9).
3.3. Single Cell Gel Electrophoresis (Comet) Assay
The alkaline single cell gel electrophoresis (comet) assay was used for analysis of sperm DNA integrity. Briefly, the mixture of sperm cell suspension with low-melting-point agarose (Sigma, USA) was layered onto a pre-coated slide. The slides were dipped in lysis buffer, electrophoresed in alkaline electrophoresis solution, and washed in neutralizing buffer. Then, the slides were air dried and stained with DNA Safe Stain (CinnaGene, Iran). Using a fluorescence microscope (Olympus BX51, Japan), 200 randomly chosen nuclei were analyzed in two slides prepared for each subject.
3.4. RNA Extraction, cDNA Synthesis, and qPCR
Semen samples were washed twice in phosphate buffer saline (Ca2+Mg2+-free; pH 7.4, 0.1 mM). Microscopic examination and treatment with somatic cell lysis buffer (0.5% Triton X, 0.1% SDS, in DEPC-treated water) were used to verify the elimination of somatic cells. Total RNA of each sperm pellets was isolated using TRIzol reagent (Life Technology, Carlsbad, CA, USA) according to the manufacturer's instructions. The extracted RNA was quantified by calculating the ratio of absorbance at 260/280 nm using a NanoDrop-2000 spectrophotometer (Thermo Fisher, Wilmington, USA). RNA was treated with DNase I (Qiagen, Germany) and reverse-transcribed into cDNA using PrimeScript® RT reagent Kit (TaKaRa, Dalian, China). Using a Real-Time PCR system (ABI Step one, Applied Biosystems, CA, USA), real-time quantitative PCR (qPCR) assays were performed triplicate for each cDNA product using SYBR Green PCR Master mix (TaKaRa, Japan). The amount of expressed PRM1 and PRM2 mRNAs were normalized against the housekeeping gene beta-actin (β-actin) and their relative expression was quantified using the ΔΔCt method. The mRNAs primers were designed using version 7.0 of Oligo primer analysis software (Molecular Biology Insights, Cascade, CO, USA) and verified for specificity using the BLAST website (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Table 1).
| Gene Symbols | Primer Sequences (5' - 3') |
|---|---|
| PRM1 | F: 5'-ACCGCCAGAGACAAAGAAGT-3' |
| R: 5'-TCTACATCGCGGTCTGTACC-3' | |
| PRM2 | F: 5'-ACCAGATCTCCCAACACCAT-3' |
| R: 5'-CAACTGCTGCCTGTACACCT-3' | |
| β-actin | F: 5'-ATTGGCAATGAGCGGTTC-3' |
| R: 5'-TGAAGGTAGTTTCGTGGATG-3' |
3.5. Statistical Analysis
The data were analyzed using SPSS software, version 16.0 (SPSS Corp., Chicago, IL, USA). Differences in the 2-ΔCt of mRNAs between groups were detected using non-parametric Mann-Whitney test. Spearman test was used to evaluate the correlations between the sperm parameters and the 2-ΔCt of mRNAs. Data were presented as median and interquartile ranges. P-values less than 0.05 were considered as statistically significant.
4. Results
4.1. Clinical Semen Parameters and Sperm DNA Fragmentation
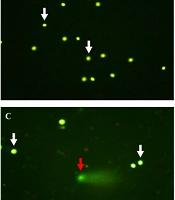
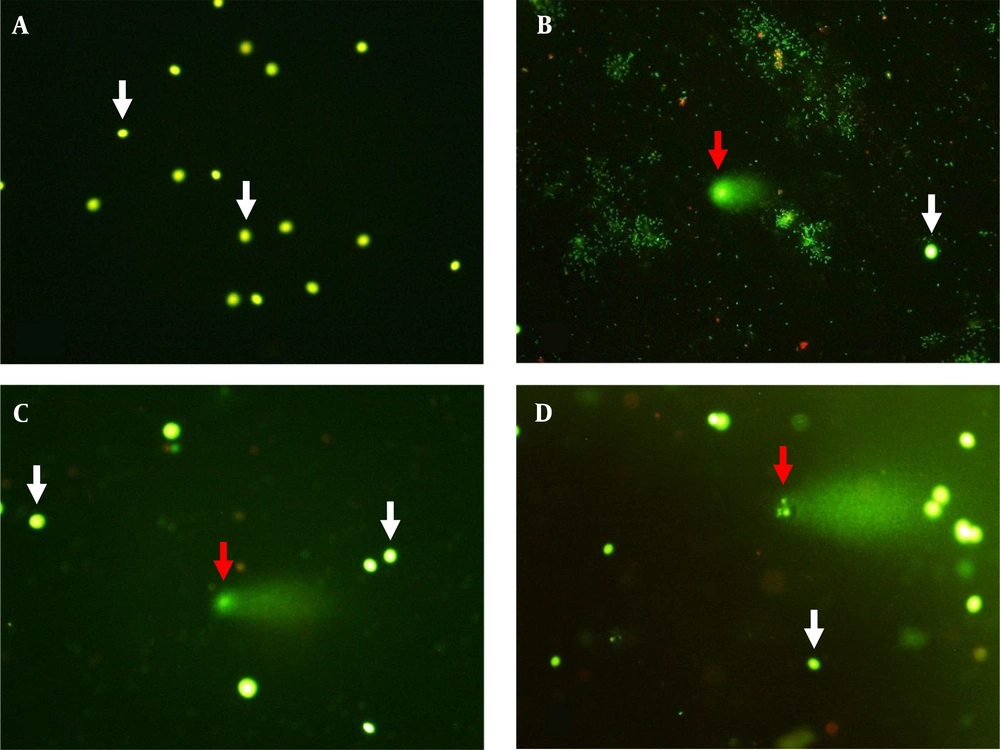
Seminal characteristics in unexplained infertile patients and healthy fertile men are presented as median and interquartile ranges in Table 2. The mean age of fertile controls (mean ± SEM: 33.3 ± 1.04, range: 27 - 39 years) showed no significant difference (P = 0.333) with unexplained infertile patients (mean ± SEM: 31.7 ± 1.15, range: 25 - 42 years). Also, no significant difference (P = 0.335) was seen in body mass index (BMI) between the patients (mean ± SEM: 26.4 ± 0.86, range: 19.5 - 31.8 kg/m2) and healthy fertile men (mean ± SEM: 25.0 ± 1.21, range: 20.8 - 34.4 kg/m2). Semen volume, total sperm motility, and sperm with normal morphology showed no significant differences between infertile men and fertile controls. However, sperm concentration and total sperm count were significantly different between infertile and fertile subjects. Moreover, in comparison with fertile subjects, a significantly higher (P = 0.001) spermatozoa with fragmented DNA (Figure 1) was seen in infertile men (Table 2).
Sperm DNA fragmentation analyzed by alkaline single cell gel electrophoresis (comet) assay (A, B, C, and D; × 400). Sperm with integrated (white arrows) and fragmented DNA (red arrows). The extent of damage to the sperm is proportional to the displacement between the genetic content of the nucleus and the resulting tail.
| Parameters | Fertile Controls (n = 10) | Unexplained Infertile (n = 17) | P Value |
|---|---|---|---|
| Semen volume (mL) | 4.2 (3.6 - 5.0) | 3.0 (3.0 - 4.5) | 0.243 |
| Sperm concentration (× 106/mL) | 80.5 (65.2 - 84.5) | 54.0 (50.0 - 71.0) b | 0.023 |
| Total sperm count (× 106) | 300.0 (213.7 - 373.8) | 216.0 (157.5 - 245.7) b | 0.009 |
| Progressive motility (%) | 45.0 (39.7 - 48.5) | 39.0 (35.0 - 47.0) | 0.074 |
| Total motility c (%) | 56.5 (48.7 - 61.2) | 55.0 (50.0 - 58.0) | 0.570 |
| Normal morphology (%) | 15.0 (12.5 - 17.2) | 12.0 (11.0 - 14.0) | 0.066 |
| DFI (%) | 12.0 (9.5 - 18.0) | 25.0 (20.5 - 28.5) b | 0.001 |
a Values are expressed as median (interquartile ranges, 25th - 75th percentile).
b P-value less than 0.05 was statistically significant.
c Progressive and non-progressive motility, DFI; DNA fragmentation index.
4.2. Sperm PRM1 and PRM2 Transcripts Levels
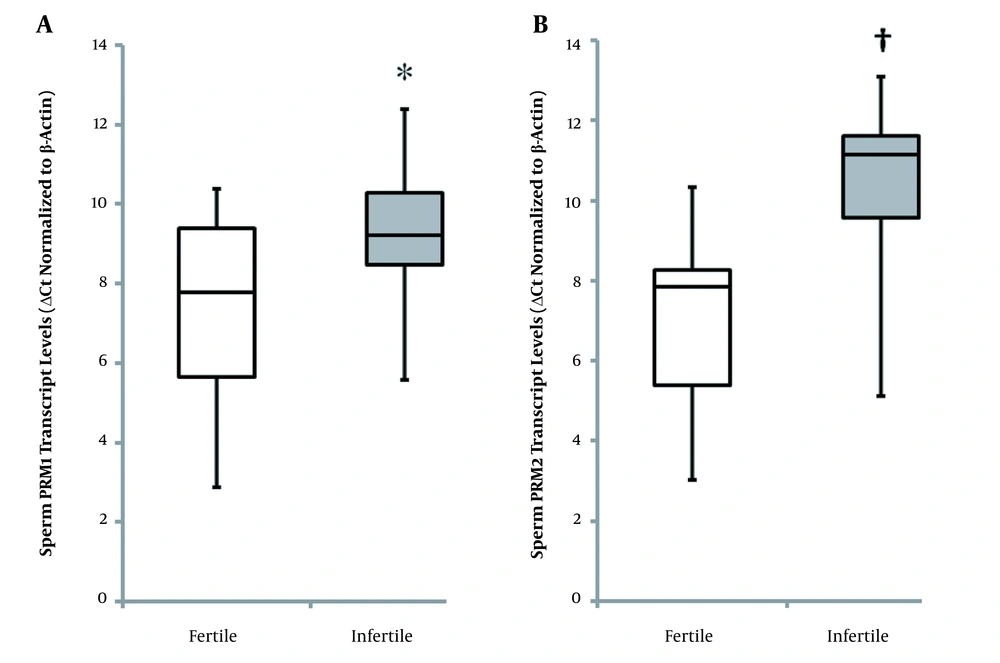
The median of sperm PRM1 ΔCt values was significantly higher (P = 0.046) in infertile patients compared with fertile individuals (Figure 2A). Lower normalized Ct values indicate higher mRNA expression levels. The level of sperm PRM1 transcript was 6.66-fold higher in fertile controls than in infertile patients. Also, significantly higher (P = 0.001) ΔCt values of sperm PRM2 were seen in unexplained infertile patients compared with healthy fertile men (Figure 2B). Level of sperm PRM2 transcript was 7.85-fold higher in healthy fertile controls than in patients with unexplained infertility. The ratio of sperm PRM1 and PRM2 ΔCt values (PRM1: PRM2 = 0.99) in healthy fertile men significantly differed (P = 0.031) from that of unexplained infertile patients (PRM1: PRM2 = 0.91). Furthermore, the ratio of sperm PRM1 and PRM2 transcript levels was significantly higher (P = 0.035) in unexplained infertile patients (3.74) compared with fertile controls (0.99).
Sperm protamine normalized Ct values in fertile controls and men of couples with unexplained infertility (box plot A and B). PRM1 and PRM2 expressions were significantly higher in healthy controls than unexplained infertile patients (* P = 0.046; † P = 0.001). Low normalized Ct values indicate high mRNA expression levels. Mann-Whitney U-test was performed. P-value less than 0.05 was statistically significant (PRM1; Protamine-1, PRM2; Protamine-2).
4.3. Correlations between Sperm Parameters, DNA Damage, and PRM Transcripts Levels
Correlations between sperm parameters, DNA damage, and protamine transcripts levels in all fertile and infertile subjects are presented in Table 3. The sperm DNA damage was significantly correlated with sperm total motility (r = -0.413, P = 0.032) and normal morphology (r = -0.424, P = 0.028). Transcripts levels of PRM1 and PRM2 in ejaculated sperm were significantly associated with total sperm count. Higher sperm DNA damage was found to be significantly associated with reduced sperm transcripts levels of PRM1 (r = -0.453, P = 0.018) and PRM2 (r = -0.492, P = 0.009). There was no significant correlation between sperm DNA damage and the ratio of sperm PRM1: PRM2 transcripts levels. A significant negative correlation was seen between the ratio of sperm PRM1: PRM2 transcripts levels (r = -0.421, P = 0.029) and sperm normal morphology.
| Parameters | Subjects (n = 27) | |||
|---|---|---|---|---|
| Total Count | Total Motility | Normal Morphology (%) | DFI (%) | |
| DFI (%) | -0.262 (0.187) | -0.413 a (0.032) | -0.424 a (0.028) | 1.00 |
| PRM1 | 0.401 a (0.038) | 0.095 (0.637) | 0.126 (0.531) | -0.453 a (0.018) |
| PRM2 | 0.467 a (0.014) | 0.123 (0.541) | 0.270 (0.174) | -0.492 a (0.009) |
| PRM1: PRM2 | -0.078 (0.699) | -0.118 (0.556) | -0.421 a (0.029) | 0.251 (0.206) |
Abbreviations: DFI; DNA fragmentation index; PRM1, protamine-1; PRM2, protamine-2.
a P-value less than 0.05 was statistically significant.
5. Discussion
It is believed that routine sperm analysis, which is used to assess fertility in the clinic, is not an effective predictor, and more reliable and clinically useful tests are needed when considering male factor infertility. Several assays have been developed to detect genetic abnormalities for providing molecular data to elucidate pathogenesis of unexplained infertility. Analysis of sperm DNA integrity is one of the proposed methods to assess sperm quality; it can explain the problem of subfertility in couples and is useful in the clinical counseling in choosing the method of assisted reproduction. This study evaluated sperm DNA damage in combination with sperm protamine transcripts contents in normozoospermic infertile men. Our findings demonstrated that men of couples with unexplained infertility exhibited significantly higher sperm DNA damage and lower sperm protamine transcripts contents than healthy fertile controls.
In this study, a higher degree of sperm with fragmented DNA was seen in men of couples diagnosed as unexplained infertile compared to healthy controls. Sperm DNA damage showed significant negative correlations with total sperm motility and normal morphology. The association between the levels of DNA damage and sperm parameters have been reported. Yuan et al. (2019) found that sperm progressive motility and normal morphology were significantly correlated with DNA damage (10). Venkatesh et al. (2011) reported that poor semen quality in idiopathic infertile men is associated with higher DNA fragmented sperm (11). However, Giwercman et al. (2003) showed that DNA damage has a low association with sperm parameters; they suggested that DNA fragmentation could be considered as an independent predictor of male fertility (12). According to our results, a DNA fragmentation index (DFI) level of 25% was obtained in unexplained infertile men compared with 12% in fertile controls. Based on previous studies, the cut-off value of 20% was determined for DFI by sperm chromatin structure assay (SCSA) (13). DFI levels of 20% or higher have been reported by Giwercman et al. (2010) in 10.5% of men with proven fertility (14). Liu et al. (2011) indicated that 37.5% of the subjects with recurrent spontaneous abortion have a DFI over 30%, and Kumar et al. (2012) found a DFI of 26% in male partners of couples experiencing idiopathic recurrent miscarriage (15, 16). Venkatesh et al. (2011) reported the cut-off value of 30.28% for DFI to discriminate idiopathic infertile men from fertile controls (11). Our findings showed that 41.1% of unexplained infertility cases had a DFI above 25 and 76.4% of them were above 20%. Bungum et al. (2007) found that in patients with a DFI > 30% the intrauterine insemination (IUI) pregnancy rate was dramatically low (at about 1.5%) compared to patients with a DFI < 30% whose pregnancy success rate was 19.0% (17).
The results of the present study also showed that spermatozoa from unexplained infertile men exhibited significantly lower levels of PRM1 and PRM2 mRNAs compared to spermatozoa from healthy fertile controls. Steger et al. (2008) suggested that PRM1 and PRM2 genes could be a useful marker for predicting male infertility (18). Kumar et al. (2012) found that the levels of PRM2 transcript were significantly lower in male partners of couples experiencing idiopathic recurrent miscarriage (16). In this study, the transcripts levels of PRM1 and PRM2 were associated with sperm motility and morphology. Various studies have shown that protamine transcripts patterns are related to seminal parameters such as sperm motility and morphology (19, 20). Akmal et al. (2016) showed that changes in protamine mRNA are related to the sperm motility (21). Lambard et al. (2004) reported that PRM1 transcript was higher in a population with poorly motile sperm than men with higher motility (19). Additionally, it has been shown that PRM1 and PRM2 transcripts ratio are different between fertile (1: 1.7) and infertile (1: 1) men (18). In the present study, the ratio of sperm PRM1 and PRM2 ΔCt values in men with unexplained infertility (0.91) was significantly different from that of fertile controls (0.99). Rogenhofer et al. (2013) found that sperm protamine transcripts ratios differ significantly between normozoospermic men (0.98) and intracytoplasmic sperm injection (ICSI) patients (Munich 0.81; Wiesbaden 0.78), while a normal ratio was seen in in vitro fertilization (IVF) patients (Hamburg 1.0; Shanghai 1.0) (8). Our results showed an association between sperm protamine transcripts ratios and normal morphology. Zini et al. (2009) and Utsuno et al. (2014) found that protamine deficiency was more frequently observed in spermatozoa with normal head morphology (22, 23). Moreover, normal ratios of protamine transcripts have been reported to be significantly associated with total motile and percent of progressively motile sperm (24). Furthermore, our results revealed that protamine transcripts levels were associated with sperm DNA damage. Nasr-Esfahani et al. (2005) reported that in ICSI patients, increased sperm DNA fragmentation was due to protamine deficiency (25). Nili et al. (2009) described the relationship between DNA damage and sperm protamine content (26). Utsuno et al. (2014) found that in protamine-deficient spermatozoa, DNA fragmentation was significantly higher than in non-deficient spermatozoa (23). Also, the relationship between the PRM1: PRM2 ratio and sperm DNA fragmentation has been reported in fertile controls, patients with clinical and subclinical varicocele, and carriers of structural chromosome reorganization (27).
In this study, changes in sperm PRM1 and PRM2 transcripts levels may explain higher sperm DNA fragmentation, which could be a possible cause of the decrease in fertility potential in men with unexplained infertility. Abnormal protamination and any changes in PRM1: PRM2 ratios, which may render the sperm more susceptible to stressors such as reactive oxygen species (ROS), lead to DNA fragmentation (5). In addition, imprinting DNA during spermatogenesis results in the transmission of epigenetic information and allows reactivation of the paternal genome following fertilization attributed to protamine (28). Simon et al. (2011) suggested that increased sperm with fragmented DNA was associated with abnormal protamination leading to reduced fertilization and pregnancy rates and poor embryo quality (29). In IVF patients, but not in ICSI patients, abnormal protamination showed a significant negative relationship with sperm DNA fragmentation, fertilization, and pregnancy rates (30). A negative correlation has been reported between protamine protein ratio and fertilization rates in ICSI patients (31). Depa-Martynow et al. (2012) found that both fertilization and embryo quality were significantly associated with protamine RNA and protein levels in couples undergoing IVF (6). Moreover, a relationship between poor sperm protamination and the development of low-quality embryos after in vitro fertilization has been reported (32). Rogenhofer et al. (2013) demonstrated that higher fertilization capacity was observed in both IVF and ICSI patients with a normal ratio of sperm protamine transcripts (8). Furthermore, de Mateo et al. (2009) reported an association between pregnancy rates and the IVF, but not ICSI success, with the protamine protein ratio (33). Sarasa et al. (2020) suggested that using the transcripts ratio of protamine as an excellent marker for sperm quality analysis could improve clinical outcomes in ICSI patients (34).
5.1. Conclusion
This study demonstrated the extent of DNA damage in ejaculated spermatozoa of unexplained infertile men in southwest Iran. Our findings indicated a DFI of 25% in unexplained infertile men compared with 12% in fertile controls. DFI levels above 25 and 20% were observed in 41.1 and 76.4% of men with unexplained infertility, respectively. Lower levels of PRM1 and PRM2 mRNAs in men with unexplained infertility were associated with low sperm motility and normal morphology. Our results also showed that PRM1 and PRM2 transcripts ratio differ between fertile (0.99) and unexplained infertile (0.91) men. In addition, the present study identified an association between degree of DNA damage and sperm protamine transcripts contents and suggested that abnormal chromatin packaging may lead to the impaired fertility potential in men of unexplained infertile couples. One of the limitations of this study was the lack of access to sufficient number of fertile and infertile subjects. Therefore, studies with a larger sample size are recommended.