1. Background
Consistent and indiscriminate use of chemical drugs causes an important phenomenon of resistance to microorganisms, and the effect of drugs is weakened or neutralized by creating this phenomenon. This resistance eventually leads to an increase in drug intake and a tendency to use compounds with newer and stronger formulations. Another disadvantage of using these drugs is the increase in their side effects, which leads to diseases that are more dangerous than the original disease (1-3).
Many plant essential oils have been reported to have significant inhibitory effects on pathogenic microorganisms. It has been shown that most plant essential oils extracted from medicinal plants have antifungal, anti-parasitic, antibacterial, and anti-viral properties. Therefore, plant essential oils have been severely screened and used in the fields of pharmacology, herbal pharmacology, medical and clinical microbiology, phytopathology, and preservation of food, fruits, and vegetables (4-6). These herbal medicines are more popular among people. These reasons have been the reason for the new wave of global studies and the introduction of antibacterial effects of various plants in recent years (7-9).
Plants have played a very important role in maintaining human health and improving the quality of life for thousands of years. Medicinal plants have beneficial properties, including antibacterial, antiparasitic, antifungal, and antioxidant properties. In recent years, plant products (secondary metabolites) have been used to treat most human and animal diseases due to their easy availability, ease of use, and fewer side effects compared to chemical products (10-12). On the other hand, plant-derived secondary metabolites, such as phenol and total flavonoids, have a strong potential to scavenge free radicals that are present in all different parts of the plant, such as leaves, fruits, seeds, roots, and skin. Therefore, given the high prevalence of chronic and erosive diseases, it necessitates using plants to provide the antioxidants needed by the body, especially plants that are high in phenol and flavonoids. Therefore, to provide the natural antioxidants needed by the body, the consumption of plants with high phenolic compounds is recommended (13-15).
Throughout human history, many infectious diseases have been traditionally treated with herbal medicines, so that today in many developing countries, herbal medicines play a major role in primary treatment (16). Because different medicinal plants have different effects on microbes and the other hand, the use of different antibiotics has, unfortunately, become common among people (17, 18), so in the present study, some medicinal plants like Cichorium intybus L., Hypericum perforatum L., Lavandula angustifolia and Thymus vulgaris L. were collected from Shahrekord. Then their extracts were evaluated on how to inhibit the growth of Bacillus cereus.
2. Methods
2.1. Herbal Materials
Medicinal plants like chicory (C. intybus L.), hyacinth (H. perforatum L.), lavender (L. angustifolia), yew (Taxus baccata), and thyme (T. vulgaris L.) were collected from Shahrekord (coordinates: 32 ° 19′32 ″ N 50 ° 51′52 ″ E) and then they were identified in the botanical laboratory of University of Zabol.
2.2. Collection of Soil Samples
Soil samples were collected from the lands of Sistan region (Zabol, southeastern Iran). Soil samples were randomly collected using a spatula at a distance of 0 to 20 cm below the soil surface, packed in sterile polystyrene bags, and transported to laboratories (19).
2.3. Purification and Maintenance of Bacterial Isolates
To obtain pure bacteria, colonies of different colors and shapes were again linearly cultured on solid nutrient medium [nutrient agar (NA)]. In order to preserve the isolates, a concentrated suspension of the young culture of each isolate was stored at minus 80°C and for long-term storage of bacteria in a liquid nutrient culture medium [nutrient broth (NB)] containing 50% glycerol, at a temperature of minus 80°C. All isolates were examined for physiological, morphological, and biochemical properties based on conventional bacteriological methods, including hot tests, pigment production, oxidase, catalase, and growth under aerobic conditions (20, 21).
2.4. Isolation of Bacillus cereus
Three bacteria were isolated from the soil of Sistan plain, and their growth characteristics (colony morphology after night incubation at 30°C and the presence or absence of precipitation) were recorded. The colony of B. cereus turns peacock blue when growing on MYP (mannitol–egg yolk–polymyxin agar). The colony of B. cereus turns pink-orange when growing on PEMBA (pyruvate, egg yolk, mannitol, bromthymol blue, and agar). Bacillus cereus colonies grown on brilliance and BCM are turquoise green (22, 23).
2.5. Data Analysis
Statistix 10 software was used for statistical calculations. Mean comparisons were performed using the least significant difference (LSD) at the 1% level, and Excel was also used to draw the shapes.
3. Results
3.1. Diameter of Inhibition Zone of Plant Extracts on Bacillus cereus
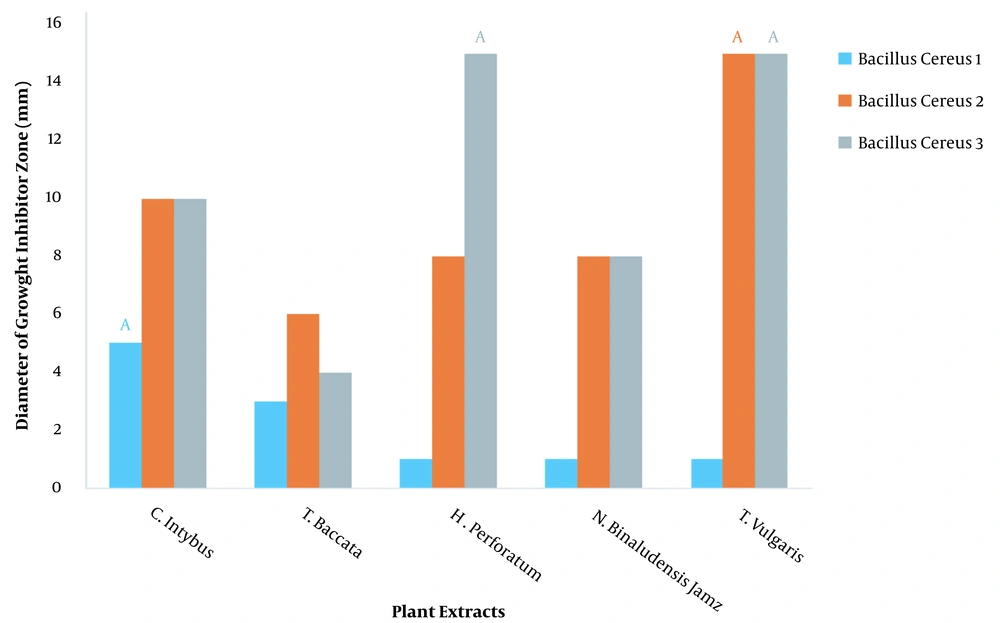
The diameter of the inhibitory zone diameter of plant extracts against Bacillus cereus at 100 ppm dilution was investigated, and it was found that different extracts had different effects on inhibiting the growth of B. cereus (P < 0.01) (Table 1). LSD test showed that thyme (T. vulgaris L.) had the highest (15 mm) effect on growth inhibition of strains 2 and 3 of B. cereus and had the lowest (1 mm) effect on growth inhibition of strain 1 of B. cereus (Figure 1).
3.2. Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of Plant Extracts on Bacillus cereus
The lowest minimumInhibitory concentration (MIC) of chicory (C. intybus L.) against B. cereus samples was 6.25 ppm, while the highest MIC was 12.5 ppm. The lowest minimum bactericidal concentration (MBC) was equal to 12.5 ppm, while the highest MBC was equal to 25 ppm (Table 2).
| Bacillus cereus strain | Taxus baccata; MIC/MBC | Lavandula angustifolia; MIC/MBC | Hypericum perforatum L.; MIC/MBC | Thymus vulgaris L.; MIC/MBC | Cichorium intybus L.; MIC/MBC |
|---|---|---|---|---|---|
| 1 | 25 - 50 | 25 - 50 | 12.5 - 25 | 12.5 - 25 | 6.25 - 12.5 |
| 2 | 25 - 50 | 12.5 - 25 | 12.5 - 25 | 3.1 - 6.2 | 12.5 - 25 |
| 3 | 25 - 50 | 12.5 - 25 | 6.25 - 12.5 | 3.1 - 6.2 | 6.25 - 12.5 |
The lowest MIC of thyme (T. vulgaris L.) against B. cereus samples was 3.1 ppm, while the highest MIC was 12.5 ppm. The lowest MBC was equal to 6.2 ppm, while the highest MBC was equal to 25 ppm (Table 2).
The lowest MIC of H. perforatum L. against B. cereus specimens was 6.25 ppm, while the highest MIC was 12.5 ppm. The lowest MBC was equal to 12.5 ppm, while the highest MBC was equal to 25 ppm (Table 2).
The lowest MIC of lavender (Nepeta binaludensis Jamzad) against B. cereus samples was 12.5 ppm, while the highest MIC was 25 ppm. The lowest MBC was 25 ppm, while the highest MBC was 50 ppm (Table 2).
The lowest and highest MIC of T. baccata against B. cereus samples was 25 ppm. Moreover, the lowest and highest MBC was 50 ppm (Table 2).
4. Discussion
In the present study, the results showed that thyme (T. vulgaris L.) extract had the greatest (15 mm) effect on inhibiting the growth of B. cereus strains. Also, the lowest MIC and the lowest MBC of thyme (T. vulgaris L.) against B. cereus samples were equal to 3.1 and 6.2 ppm, respectively.
The effect of essential oil of Zataria multiflora on B. cereus growth probability in heart and brain broth environment and the results showed that the logarithm of B. cereus growth probability decreased with a slight increase in essential oil concentration. Thyme essential oil in small concentrations has a significant inhibitory effect. This effect has also increased significantly with decreasing storage temperature so that complete growth cessation (logarithm of growth probability equal to -4.54) was obtained during 43 days of study at a concentration of 0.03 and 0.045% of essential oil at 10°C (24). Zataria multiflora essential oil and storage temperature were studied on B. cereus ATCC11778 in commercial barley soup, and it was concluded that different concentrations of essential oil on the growth rate of bacteria were statistically significant. It was also suggested that Z. multiflora essential oil, as a natural plant flavor, has a protective effect against B. cereus bacteria in soup and can be used as a preservative for some foods (25). In the present study, it was found that thyme had the greatest effect on Bacillus cereus, which was similar to the presented research.
The essential oils of thyme, savory, rosemary, mint, and peppermint have been studied on five gram-positive bacteria compared to three antibiotics. The results showed that thyme and savory essential oils have a great inhibitory effect on the growth rate of food bacteria. Twenty microliters of them with the greatest inhibitory effect increased the shelf life of food. Compared to antibiotics and essential oils, safflower essential oil has the most inhibitor effect on enterococci. Thyme has been shown to be more effective in inhibiting B. cereus, Listeria monocytosis, and Staphylococcus aureus (26). In the present study, it was found that thyme had the greatest effect on B. cereus, which was similar to the present study.
Bacterial and fungal strains tested (B. subtilis, S. aureus, S. epidermidis, Pseudomonas aeruginosa, Escherichia coli, Mycobacterium smegmatis, and fungal strains, e.g., Candida albicans and C. vaginalis) were sensitive to thyme essential oil. They showed a very effective antibacterial and antifungal activity with a MIC of 75 to 1100 μg/mL and 80 and 97 μg/mL, respectively (27). In the present study, the MIC was from 3.1 to 25, which indicates the effect of thyme extract on B. cereus in relation to the mentioned microbial substances.
The combined effects of monolaurin and essential oils of peppermint (M. pulegium L.) and peppermint (Mentha spicata L.) on B. cereus and E. coli O157: H7 have been investigated in vitro. The results showed that the most effective antimicrobial agents on B. cereus were monolaurin, a combination of monolaurin with peppermint essential oil, and a combination of monolaurin with peppermint essential oil. The least effective factor on it was mint essential oil (28). In the present study, thyme extract without any active ingredients or antibacterial auxiliaries could have the greatest effect on inhibiting the growth of B. cereus, which shows the higher growth power of thyme than peppermint.
Medicinal plants are one of the most prominent plants in the field of allelochemicals due to their secondary metabolites. On the other hand, the demand for medicinal compounds has increased, but some of these plants face problems because they have limited natural habitats, and depending on the environmental and geographical conditions of the plant, their collection is associated with obstacles. Researchers have focused on the use of biotechnological solutions to increase the production and productivity of medicinal plants due to low concentrations of medicinal compounds in the plant, limited natural resources, increasing destruction of forests, pastures, and green space, extinction of diverse plant species, problems associated with domestication and crop cultivation of these plants biotechnology is able to increase the efficiency of plants as renewable sources for drug production by using various sciences such as biology, biochemistry, genetics, etc. and by using cell culture strategies, organs, genetic engineering, and molecular markers (29).
Antioxidant properties of Origanum vulgare L. M. pulegium and thyme essential oil by testing its inhibitory effect on DPPH radical activity and antibacterial activity against one gram-positive strain (B. cereus CCM 2010) and two Gram-negative strains (P. aeruginosa CCM 1960 and E. coli CCM 3988) ) and concluded that thyme essential oil showed more antibacterial activity against E. coli at concentrations of 0.75 and 0.375 mg/mL. Thyme essential oil has shown less anti-DPPH activity compared to ascorbic acid (30). Compared to the present study, it can be said that essential oils and extracts, as well as the type of solvent, have different abilities to extract beneficial substances and consequently will have different effects against bacteria.
The essential oils from T. hyemalis (thymol) followed by T. hyemalis (carvacrol), T. zygis (thymol), and T. vulgaris possesses antimicrobial properties, and are a potential source of antimicrobial ingredients for the food industry (31). Thyme was collected from four anthogenetic stages, and it was found that the essential oils had significant bacteriostatic activity against the tested microorganisms. This activity is more pronounced against gram-positive bacteria. Thyme essential oil in whole flowers was the most effective substance in preventing the growth of microbial species. It was also shown that the essential oils tested by direct contact have good antibacterial activity, which seems to be more common against Gram-negative bacteria. Most strains are almost completely inactive, but E. coli has been the most sensitive species (32), Representing different effects of a plant species against bacteria. Different strains of a bacterium may even have different levels of resistance and sensitivity to a plant extract or essential oil.
4.1. Conclusions
Undoubtedly, the use of medicinal plants has been the oldest human approach to treating diseases. In the struggle for the development of all human civilizations, there has always been a close and shoulder-to-shoulder relationship between man and plants. Due to the proven antimicrobial effects of various medicinal plants in numerous studies, drug resistance caused by pathogens, and side effects of chemical antimicrobials, the approach of scientific research to natural resources has increased greatly in recent decades (33, 34). On the other hand, the results of this study suggest that thyme (T. vulgaris L.) can be used to treat infections caused by B. cereus. However, testing in the living system is necessary to evaluate the possible toxicity of extracts, especially thyme extract (T. vulgaris L.), to investigate (in vivo) their properties and effects, and to obtain appropriate concentrations of these extracts for use in living organisms. Extensive use of T. vulgaris L. by local people in Iran as a general disinfectant (mouth, throat, skin) to treat respiratory infections and gastrointestinal disorders can be related to antimicrobial activity. This study confirms the traditional Iranian preparation of T. vulgaris L. as oil and leaf powder. The use of raw medicine as a leaf powder includes all the volatile and non-volatile active components of the plant. Thymus vulgaris L. may be considered a promising natural source for nutrients and herbal medicines and may be used as a natural antibacterial or as a synergistic agent with antibiotics.