1. Background
Obesity is linked to several diseases, including non-alcoholic fatty liver disease (NAFLD), insulin resistance (IR), and type-2 diabetes (T2DM) (1). Under this condition, hepatic de novo fatty acid synthesis and uptake from plasma exceed oxidation causing fat accumulation in the liver. NAFLD is associated with defects in the activity of insulin-dependent lipogenic proteins such as Sterol regulatory element-binding proteins (SREBPs) (2). It is also associated with defects in insulin signaling transduction through insulin-mediated insulin receptor substrates (IRS). SREBPs are major proteins involved in the regulation of hepatic cholesterol and fatty acids synthesis (2). SREBP1c isoform seems to play a key role in lipid synthesis induced by insulin in response to dietary intake (3). Secondary to hyperinsulinemia, SREBP1c is likely to increase under obesity and IR. Elevated SREBP1c expression in leptin-deficit mice also partly explains its role in hepatosteatosis and IR (4). SREBP1c also inhibits insulin receptor substrate (IRS-2) transcription in murine hepatocytes (5) that indicates its mediatory role in hepatic IR. It seems to be the consequence of impaired signaling of insulin receptors and activation of insulin-dependent pathways. Hence, impaired insulin signaling pathways such as a decrease in insulin-receptor kinase activity and insulin receptor substrates may link IR and obesity (6). Upon activation by insulin, IRS-2 associates with several proteins such as Akt that regulate metabolism (7). Hence, a decrease in IRS-2 may be responsible for hepatic IR caused by obesity and T2DM (7).
Regular physical activity and dietary interventions are known to prevent obesity and its related complications (8). HIIT program has been shown to bring about various health benefits, including improved IR, lipid profile, and hepatic function (9). However, the mechanisms by which HIIT affects hepatic IR are not extensively delineated. HIIT has been shown to lower blood glucose, hepatic lipogenesis and fat accumulation, beta-oxidation, and PPAR activity (10). Exercise-induced insulin action may be mediated by IRS-2 activity as Howlett et al. (11) suggested that insulin signaling is increased immediately following exercise that can enhance IRS-2-associated PI3K activity. Furthermore, it has been indicated that SREBP1c inhibits transcription of IRS-2 in hepatocytes (5). Overexpression of SREBP1c and obesity decreases expression of IRS-2 mRNA and protein (12). Thus, IRS-2 and SREBP1c may contribute to the effects of HIIT on hepatic IR in obese rats.
Coenzyme Q10 (CoQ10) has also been suggested to exert glucose-lowering effects and improve IR (13). Zhang et al. (13) suggested that treatment with CoQ10 is relatively safe that can assist glucose control and improve lipid profile in patients with T2DM. Shen and Pierce (14) also indicated that elevation in CoQ10 content of mitochondrial inner membrane is associated with enhanced pancreatic β cells function that is mediated by glycerol-3-phosphate dehydrogenases. In spite of reports on the benefits of CoQ10 on glycemic control, our understandings of the mechanisms to explain how Co10 improves glucose regulation remains to be elucidated. Furthermore, combined effect of HIIT and CoQ10 supplementation on proteins involved in hepatic IR and lipogenesis is not investigated as yet.
2. Objectives
This study aimed to investigate the effect of 12 weeks of high-intensity interval training (HIIT) and Coenzyme Q10 supplementation on hepatic IRS-2 and SREBP1 proteins in obese male rats.
3. Methods
3.1. Animals
An experimental study was conducted and 48 male Wistar rats (age: 21 days; weight: 40 ± 5 g) were purchased from the Pasteur Institute Animal Care Center (Karaj, Iran). The rats were housed in an ambient temperature of 22 ± 2°C and humidity of 50% ± 5% under 12 h light-dark cycle. They were provided with standard chow and water ad libitum.
3.2. Diet and Obesity Induction
Following a week-long acclimatization, eight rats were randomly assigned to the non-obese control group (NC), and 40 remainings were fed a high-calorie diet (high-fat chocolate milk, 960 Kcal/ L, 14% protein, 20% fat, and 68% carbohydrates) for six weeks. Weekly assessment of weight and chocolate milk consumption was carried out throughout the study. Following a 6-week administration of the high-calorie diet and after obesity was confirmed by Lee index ((weight-3/ nasoanal length) × 1000), eight rats were randomly assigned to the baseline obese-control group (BC). Both control groups were sacrificed at age 11 weeks before the main interventions.
3.3. Exercise Testing and Training
Maximum running speed was determined by a standard exercise protocol. The test began with 10 m.min-1 with a gradual increase of 3 m.min-1 every two minutes until exhaustion. Following exercise testing, the rats were randomly assigned to matched groups of HIIT (n = 8), CoQ10 (n = 8), HIIT + CoQ10 (n = 8), and control (n = 8). HIIT program consisted of two min running at 85% - 90%speedmax interspersed with two min running at the 45% - 50%speedmax underwent five sessions per week for 12 weeks (Table 1).
| Week of Training | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| High-intensity interval (85% - 90% speedmax) | ||||||||||||
| Speed, m/min | 26 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 |
| Time, min | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Low-intensity interval (45% - 50% speedmax) | ||||||||||||
| Speed, m/min | 10 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Time, min | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Numbers of repetitions | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Total workout time (min /day) | 20 | 24 | 28 | 32 | 36 | 40 | 44 | 48 | 52 | 56 | 60 | 64 |
Details of HIIT Program
3.4. Supplementation
Prior to and after each exercise session, the experimental groups were either gavage-fed 500 mg.kg-1 CoQ10 supplement (Golden Life, Iran) or n equal volume of normal saline as the placebo.
3.5. Tissue Preparation
All animals were anesthetized with an intraperitoneal injection of ketamine (90 mg.kg-1) and xylazine (10 mg.kg-1) and were then sacrificed 48 h following the last exercise session. The liver tissue was removed and transferred to cryotubes to be stored in liquid nitrogen under -80°C (15).
3.6. Electrophoresis and Western Blot Analysis
Protein lysates were isolated using lysis buffer supplemented with complete protease inhibitor cocktail and centrifuged at 12,000 × g for 15 min at 4°C. The protein concentration of the supernatant was determined by the Bradford method. Proteins were separated using SDS-polyacrylamide gel electrophoresis using 8% - 12% denatured ready gel and transferred onto a polyvinylidene difluoride (PVDF) membrane (Sigma-Aldrich, Germany). The membrane was blocked for 1 h in 5% BSA in tris-buffered saline and 0.1% Tween 20 (TBST) to block non-specific bindings. Subsequently, blots were incubated overnight at 4°C with primary antibodies: β-actin (sc-47778), SREBP1C (sc-365513), and IRS-2 (sc-390761). The membrane was then washed three times and incubated with the appropriate secondary antibody for 1 h at room temperature in 5% milk in TBST. Protein bands were visualized with an enhanced chemiluminescence (ECL) reagent and radiographic film (Fujifilm, Tokyo, Japan) quantified by densitometry analysis with Image J software (National Institute of Health, Bethesda, Maryland, USA) (16). Then. the density of the target protein bands was normalized against beta-actin control loading. Finally, the results were presented as relative density (compared to the NC group).
3.7. Statistical Analysis
Data were analyzed using SPSS software version 19. Normal distribution of data was confirmed by Shapiro-Wilk test, and statistical analyses were performed by independent t-test and two-way analysis of variance. Data were presented as mean ± SD, and statistical significance was P < 0.05.
4. Results
Baseline comparisons (NC vs. BC) indicated a significant increase in weight (Mean ± SD: 158.6 ± 22.2 Vs. 302.4 ± 15.3, P = 0.001) and Lee index (mean ± SD: 284.2 ± 35.2 vs. 352.0 ± 29.7, P = 0.0001) following obesity induction. Total body weight and Lee index decreased following HIIT program (P = 0.001, η2 = 0.49; and P = 0.004, η2 = 0.37, respectively) (Table 2).
| Variables | Group | |||||
|---|---|---|---|---|---|---|
| NOC | BOC | Control | HIIT | CoQ10 | HIIT + CoQ10 | |
| Age, wk | ||||||
| Initial | 3 | 3 | 3 | 3 | 3 | 3 |
| After obesity induction | 11 | 11 | 11 | 11 | 11 | 11 |
| After intervention | - | - | 24 | 24 | 24 | 24 |
| Weight, g | ||||||
| Initial | 40 ± 5 | 40 ± 5 | 40 ± 5 | 40 ± 5 | 40 ± 5 | 40 ± 5 |
| After obesity induction | 158.6 ± 22.2 | 302.4 ± 15.3a | 294.43 ± 11.67 | 300.22 ± 10.42 | 298.89 ± 12.57 | 301.12 ± 9.94 |
| After intervention | - | - | 326.1 ± 18.3 | 304.2 ± 17.5 | 331.1 ± 22.1 | 307.3 ± 19.8 |
| Lee index | ||||||
| After obesity induction | 284.2 ± 35.2 | 352.0 ± 29.7a | 349.47 ± 33.17 | 343.07 ± 36.45 | 351.57 ± 28.88 | 343.59 ± 35.2 |
| After intervention | - | - | 312.7 ± 27.6 | 304.0 ± 21.1 | 314.3 ± 30.7 | 306.3 ± 24.5 |
| Chocolate milk intake, mL. day-1 | ||||||
| During obesity induction | - | 18 ± 4.5 | ||||
Characteristics of Rats in Different Groups
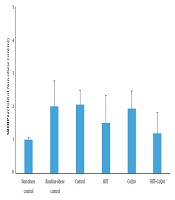
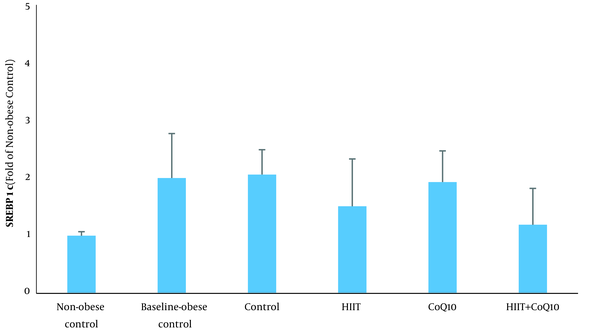
Data analysis revealed that hepatic SREBP1c protein content was higher in the BC group compared to the NC group (P = 0.012). Analysis indicated that HIIT program significantly decreased hepatic SREBP1c content (P = 0.007). However, CoQ10 supplementation had no significant effect on hepatic SREBP1c protein levels (P = 0.322). In addition, there was no significant interaction between treatments (HIIT × CoQ10) on hepatic SREBP1c protein level (P = 0.677) (Figure 1).
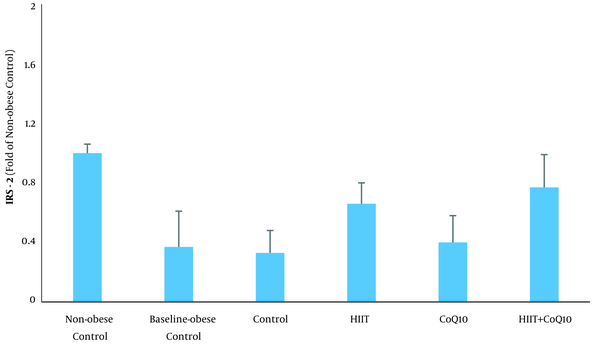
Analysis revealed an elevation of IRS-2 protein in the BC group compared to the NC group (P = 0.0001). HIIT intervention increased IRS-2 content (P = 0.0001), but no significant changes were observed following CoQ10 supplementation (P = 0.154). In addition, there was no significant interaction between treatments (HIIT × CoQ10) on hepatic IRS-2 protein level (P = 0.577) (Figure 2).
5. Discussion
In this study, a 6-week long administration of a high-calorie diet resulted in approximately 90% weight gain (NC Vs. BC), indicating the efficacy of this regimen to induce obesity. In contrast, HIIT, but not CoQ10 supplementation, decreased total body weight; yet, it was remained elevated when compared to the NC group. HIIT is a time-efficient exercise protocol that increases energy expenditure during and following exercise (17). Given the elevated energy expenditure by HIIT protocol, a reduction in weight was expected in this study.
We found a marked elevation in hepatic SREBP1c protein level in obese group compared with lean control. It is consistent with Pettinelli et al. (18), who reported a significant increase of SREBP1c in obese patients. The expression of SREBP1c is decreased in insulin-deficient state but is enhanced by feeding with a high-fat diet in animal models of IR (3). SREBP1c activates the transcription of various genes encoding enzymes, mediating cholesterol and fatty acids synthesis. Feeding state activates mTORC1 signaling and induces insulin-dependent SREBP1c mRNA expression. Insulin signaling is transferred through PI3K, downstream of mTORC1 and PKC-λ, that is required for activation of SREBP1c (3). Obese patients also exhibit upregulated PPAR-γ in the liver that is suggested to be an additional reinforcing lipogenic mechanism to SREBP1c (19).
We found that hepatic IRS-2 protein decreased by obesity that is consistent with some previous studies (12). All isoforms of hepatic IRS contribute to IR, glucose intolerance, and hyperglycemia in fasted and fed state (20). In addition, IRSs contribute to insulin signaling transduction as IRS-deficit mice develop IR. Similar to SREBP1c, IRS-2 is also affected by fasted and fed state as 16-hour fasting increased IRS-2 mRNA expression by 3.5 folds (20) while hyperinsulinemia under fed state and refeeding reduced liver IRS-2 (20). It seems to be a link between SREBP1c and IRS-2 in that IRS-2 potentially regulates lipogenic genes. The IRS-2 promoter includes a connection site to SREBP1c, and a relative decrease in IRS-2 leads to upregulation of SREBP1 (20). In addition, insulin-dependent stimulation of SREBP1c negatively regulates IRS-2 expression (12, 20). Hepatic IRS-2 deficit is associated with upregulated SREBP1c and elevated fatty acid synthesis (20). Also, IRS-2 is regulated by insulin via mTOR pathway. Insulin induces ubiquitination and proteasomal degradation of IRS-2 via the PI3K-Akt-mTOR pathway (12) IRS-1 and IRS-2 knockout mice exhibit activated mTORC1 signaling that induces SREBP1c mRNA expression through insulin signaling pathway (21). In this study, the elevation of hepatic SREBP1c was coupled with decreased IRS-2 in obese rats that may imply a link between hepatic IRS-2 and SREBP1c under obesity.
On the other hand, HIIT decreased hepatic SREBP1c protein levels. Consistent with this finding, Hedayati Katouli et al. (22) reported that aerobic exercise reduced SREBP1c in obese rats. Kalaki-Jouybari et al. (23) also indicated that HIIT program reduced hepatic SREBP1c in diabetic rats. HIIT is established to decrease circulating fatty acid concentration and improve glycemic control that may be due to the decreased hepatic SREBP1c protein (24). Amri et al. (24) showed that HIIT is more effective than endurance training to improve glycemia in diabetic rats. Insulin lowering effect of HIIT may also partly explain decreased hepatic SREBP1c protein level. Besides, it has been indicated that miR-122, an anti-lipogenic microRNA, decreases during obesity and upregulates hepatic SREBP1c. Kalaki-Jouybari et al. (23) suggested that elevated miR-122 following HIIT may explain decreased SREBP1c in diabetic rats. PPAR-γ is also suggested to be involved in upregulation of SREBP1c under obesity and a high-fat diet intake (19). Motta et al. (10) indicated that HIIT downregulates PPAR-γ and lipogenesis that can partly explain reduced hepatic SREBP1c. The changes in SREBP1c following HIIT program may also be mediated by activation of extracellular-signal-regulated kinase-1 and 2 (25).
In line with a number of studies, we found that HIIT increased hepatic IRS-2 in obese male rats. A review of the literature indicates that exercise training upregulates the expression of isoforms of IRS. For instance, Kirwan et al. (26) showed that endurance training increases IRS-1 and related activity of PI3K. PI3K activity is enhanced immediately following exercise, downstream to IRS in insulin signaling transduction (11). Howlett et al. (11) suggested that the increase in PI3K activity can be explained by enhanced IRS-2 following exercise. Although the effects of acute exercise may be transient, regular exercise potentially induces physiological adaptations that can promote IR. Since IRS-2 is tightly regulated by insulin, decreased circulating levels of insulin by HIIT are supposed to be the major cause of elevated IRS-2 in this study. Furthermore, increased IRS-2 following 12 weeks HIIT program is likely to be explained by reduced SREBP1c as it negatively regulates IRS-2 expression (12, 20). Ide et al. (5) suggested that SREBPs inhibit IRS-2 mediated insulin signaling in the liver.
In spite of positive effects of HIIT on SREBP1c and IRS-2, CoQ10 supplementation had no remarkable effect on these variables and had no synergistic effect along with HIIT. To the best of our knowledge, this is the first study investigating the combined effect of CoQ10 and HIIT on hepatic SREBP1c and IRS-2 in obese rats that raises a challenge to compare the findings with other research. Coenzyme Q10 has been shown to promote glycemic control and IR in patients with T2DM (13, 14). It is speculated that elevation of CoQ10 content in the mitochondrial inner membrane improves glycerol-3-phosphate dehydrogenase (G3PD) activity, a key enzyme affecting β cells’ function (14). Despite promising findings on the benefits of CoQ10 to assist glycemic control (14, 27), understandings on the mechanisms of effect of CoQ10 on glycemic indices remain unclear.
The results of the present study show that the impact of CoQ10 on IR is less likely to be mediated by hepatic SREBP1c and IRS-2 proteins. Therefore, any possible effect of CoQ10 on glycemia and IR may be mediated by other pathways. This study includes some limitations that should be acknowledged. As noted earlier, SREBP1c and IRS-2 are tightly regulated by insulin, and exercise interventions markedly alter circulating levels of insulin and IR. However, we neither assessed circulating levels of insulin nor IR in this study. Metabolic processes in the liver are strongly affected by circulating free fatty acid and glucose that were not measured in this study. Measurement of insulin, free fatty acid, and glucose would have provided further mechanistic insights into our findings.
5.1. Conclusions
Obesity enhanced hepatic SREBP1c, while decreased IRS-2 protein levels. Moreover, 12-week HIIT intervention decreased hepatic SREBP1c, whereas elevated IRS-2. However, CoQ10 had no significant effect on obesity-induced alterations in hepatic SREBP1c and IRS-2. Based on the findings, we conclude that HIIT is responsible for the alleviation of changes in SREBP1c and IRS-2 and CoQ10 have no synergistic effect along with HIIT.