1. Background
Tissue engineering aims to develop biological substitutes that replace, restore, and maintain diseased or damaged tissues (1, 2). The three critical materials in the field of tissue engineering are cells, soluble signaling factors, and scaffolds. One of the main challenges in tissue engineering is fabricating and designing suitable scaffolds (3). Scaffolds play an essential role in tissue repair and regeneration by acting as a three-dimensional (3D) support structure that promotes cell adhesion, migration, differentiation, and proliferation until the tissues are completely restored (4, 5). The ideal scaffold should mimic the native extracellular matrix (ECM) to provide a suitable environment to chemically inform or physically guide cell response, and consequently, promote new tissue formation (6, 7). In this regard, a simple, flexible, and inexpensive method for preparing nanofibrous scaffolds is electrospinning (8).
The unique potential of scaffolds to mimic the structure and biological functions of natural ECM at the nanometer scale is one of the main advantages of using the electrospinning technique (9). Electrospun scaffolds can provide an appropriate environment for cellular morphogenesis and development due to their similarity to the fibrous structure of natural ECMs (10, 11). Different natural and synthetic polymers have passed the electrospinning process as bio-mimetic and temporal substrates (12). An essential step in the success of tissue regeneration is choosing the desired biomaterials for scaffold engineering (13). Moreover, key requirements in the selection of biomaterials for scaffold fabrication are biodegradability and biocompatibility. The scaffolds that are fabricated with biodegradable polymers can be absorbed by the surrounding tissues, and no additional surgery is required to eliminate them from the biological environment of the human body (14-16). The preparation of tissue engineering scaffolds is done by electrospinning of gelatin with poly(vinylalcohol) (PVA). Due to having a simple chemical structure and containing numerous polar alcohol groups, PVA is recognized as a hydrophilic polymer. Its unique mechanical properties have made it a biodegradable material widely used in tissue engineering (17, 18).
Natural polymers, such as gelatin which is derived from collagen, are also broadly used in tissue engineering. Due to its biocompatibility and ability to provide a better environment for cell growth and attachment, gelatin can be used as a scaffold material for substituting a natural ECM (19). Other excellent characteristics of gelatin include low antigenicity, bio-affinity, hydrophilicity, and biodegradability (20). Composite fibrous scaffolds containing both natural polymers for cellular attachment and synthetic polymers for mechanical support help improve scaffold properties, including biocompatibility and biodegradation (21, 22).
2. Objectives
The purpose of this study was to provide an appropriate micro-environment for the adhesion and proliferation of hUC-MSCs over the glutaraldehyde/methanol crosslinked PVA/GE nanofibrous scaffold to be differentiated into osteoblast cells.
3. Methods
3.1. Materials
In the supporting information, a detailed description of the materials used in this study is provided.
3.2. Instruments
Please refer to supporting information to see the instrumentation details.
3.3. Fabrication of Crosslinked PVA/GE Electrospun Scaffold
The PVA/GE scaffolds were fabricated using the electrospinning process, and then they were crosslinked by GA in two steps (for more details, see supporting information).
3.4. Contact Angle Test
The experimental method for determining the contact angle of the prepared scaffolds is described in the supporting data.
3.5. Isolation of hUC-MSCs from Umbilical Cord Wharton’s Jelly, Cell Culture, Differentiation, and MTT Assay
A full description, including the isolation of hUC-MSCs from umbilical cord Wharton’s Jelly, cell culture, differentiation, and MTT assay, is provided in the supporting data.
3.6. Degradation Measurements and Swelling Test of PVA/GE Nanofibrous Scaffold
Some physicochemical features of crosslinked PVA/GE scaffold are evaluated by degradation, pH changes, and swelling tests (see supporting information for more details).
4. Results
4.1. Morphological Study of PVA/GE Nanofibrous Scaffolds
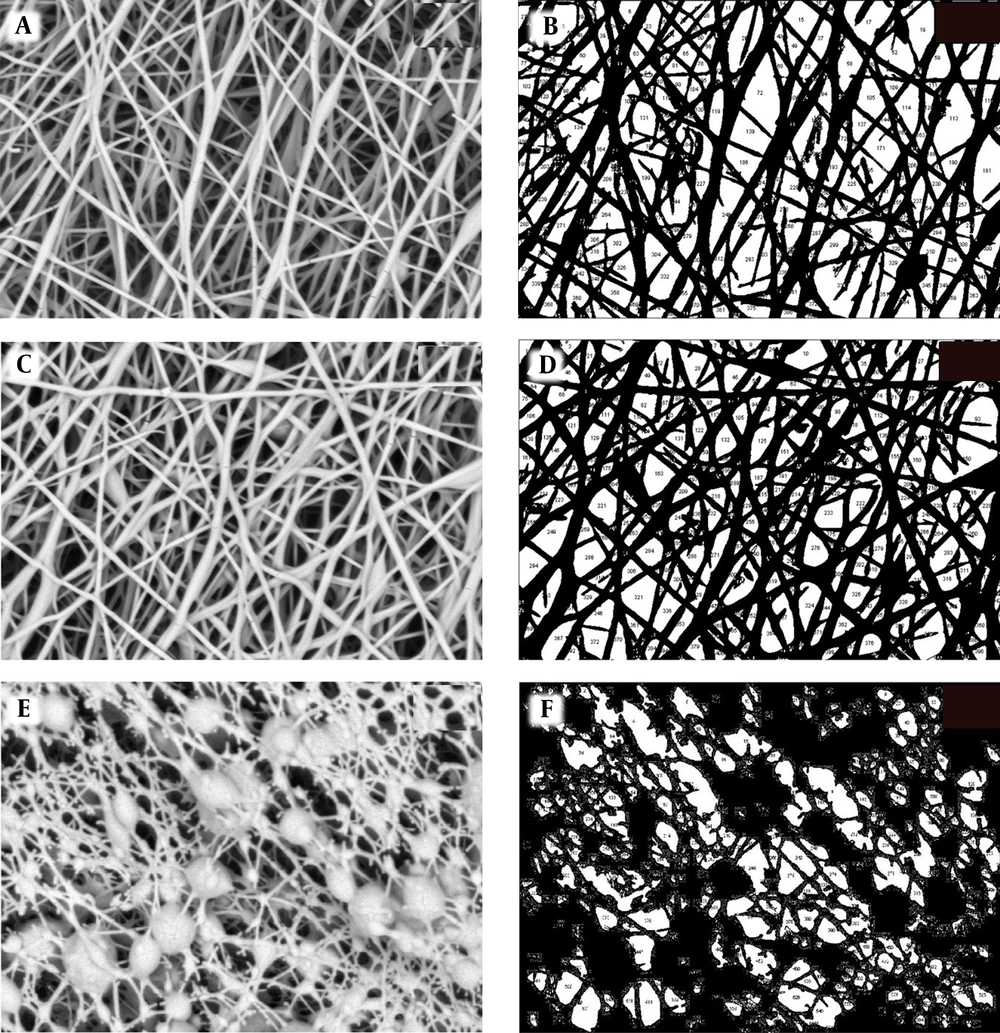
Scanning electron microscopy micrographs at 30000× magnification were used to evaluate the morphology of PVA/GE scaffolds in three different situations (Figure 1A, C, and E). The obtained images indicated the PVA/GE 50:50 nanocomposite was formed with interconnected pores and random orientation. Further investigation was carried out by Image J software, which shows the diameter of nanofibers and porosity percentage of scaffolds (Figure 1B, D, and F). Before crosslinking, the PVA/GE diameter was in the 80-90 nm range, and the porosity percentage was 39.5% (Figure 1A and B). We performed two steps of crosslinking to the water-soluble property of the scaffolds to be useful for the bone tissue engineering application. Hence, we treated scaffolds on GA vapor (20%) for 24 h in the first step of crosslinking. The treatment relatively stabilized the scaffolds against water dissolution, but fiber diameter and porosity percentage did not change significantly (Figure 1C and D). The GA/methanol-treated scaffolds were stabled in buffer solution, and their water resistance properties was significantly enhanced. It was observed that the porosity percentage of the scaffolds was 27.5% (Figure 1E and F). The porosity of the crosslinked scaffold by GA/methanol decreased. However, these crosslinked nanocomposite scaffolds had a good surface to volume ratio, which is beneficial for hUC-MSCs proliferation and migration.
4.2. FT-IR and TGA Analysis
Fourier-transform infrared spectroscopy (FT-IR) spectra are known as valuable tools to identify the presence of specific functional groups or chemical bonds in a compound of interest. Appendix 1A in the Supplementary File shows the FT-IR spectra of PVA, gelatin, and crosslinked PVA/GE fibrous scaffolds (see supporting information for more details). The TGA curves of the PVA, gelatin, crosslinked PVA/GE, and non-crosslinked PVA/GE nanocomposites are presented in Appendix 1B in Supplementary File (see supporting information for more details).
4.3. Contact Angle Measurement
The results of wettability studies are described in the supporting data.
4.4. Degradation Test, pH Changes, and Swelling Behavior of Crosslinked PVA/GE Nanofibrous Scaffold
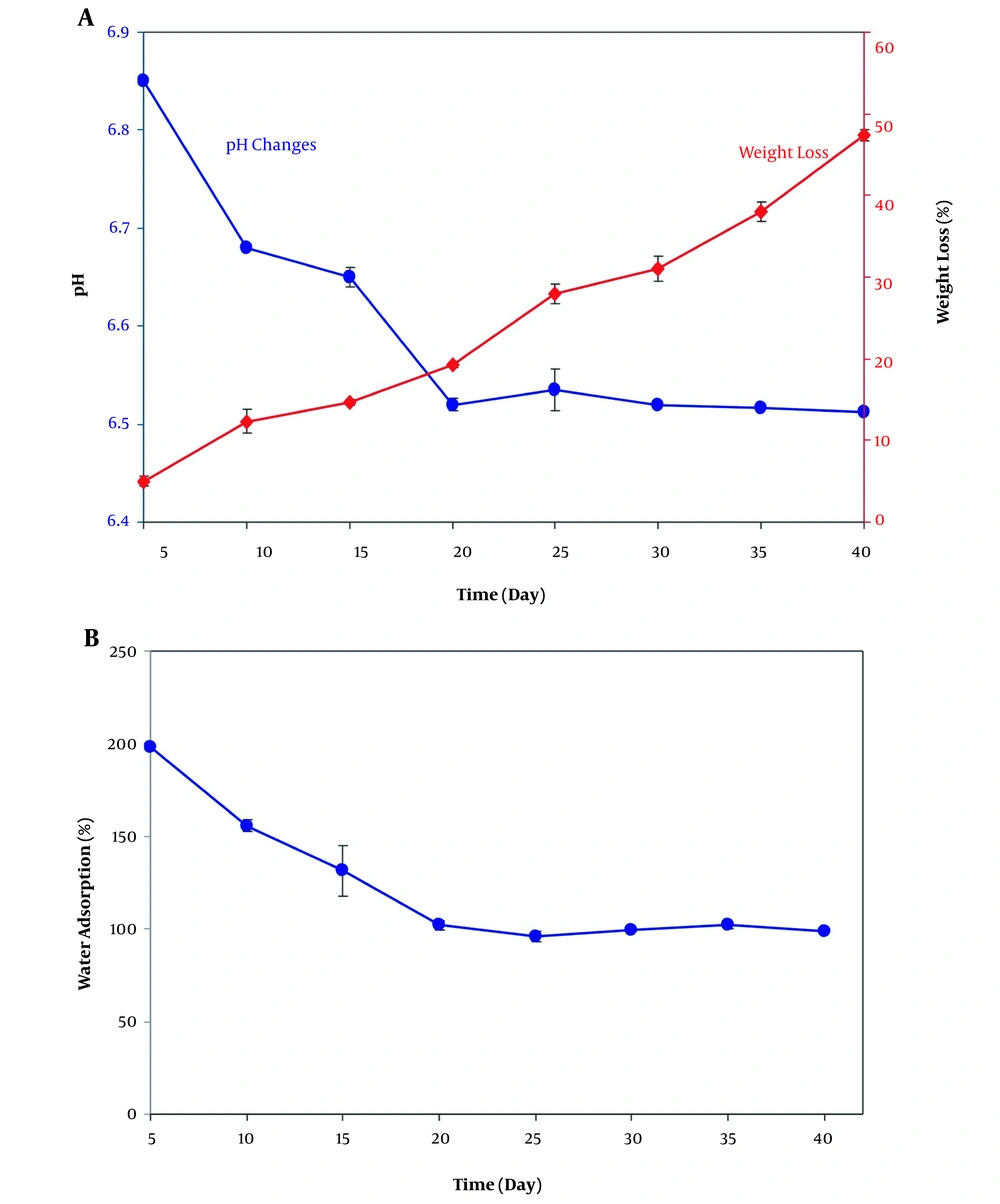
In this test, degradability and pH changes over the degradation course of the crosslinked scaffold were evaluated by measuring the weight loss and pH values as a function of incubation time in phosphate-buffered saline (PBS) solution at 37°C (Figure 2A). The weight loss curve indicated that the maximum weight loss percentage of scaffolds was 47%, which occurred on day 40. Also, the pH changes of PVA/GE nanocomposite scaffolds in PBS solution at 37°C was studied (Figure 2B). As can be seen, the pH values dropped during scaffold degradation through 40 days. The significant decrease in pH values occurred during the first 20 days of degradation by acid production. No significant changes were perceived at the end of the study period and showed the pH value of 6.5 after 40 days of incubation. It was clear that the release of acidic agents decreased through the last days of degradation (23). The results of water absorption tests of crosslinked PVA/GE scaffold illustrated that the capacity of the scaffold to absorb water decreased during the 40 days of immersion in PBS (Figure 2B). The maximum water absorption rate took place during the initial incubation period and was 198%. The rate of water uptake reduced in the remainder of the test and reached 100%. After 20 days, PVA/GE nanofibers reached water absorption equilibrium.
4.5. Cell Morphology Isolated from Human Umbilical Cord Mesenchymal Stem Cells
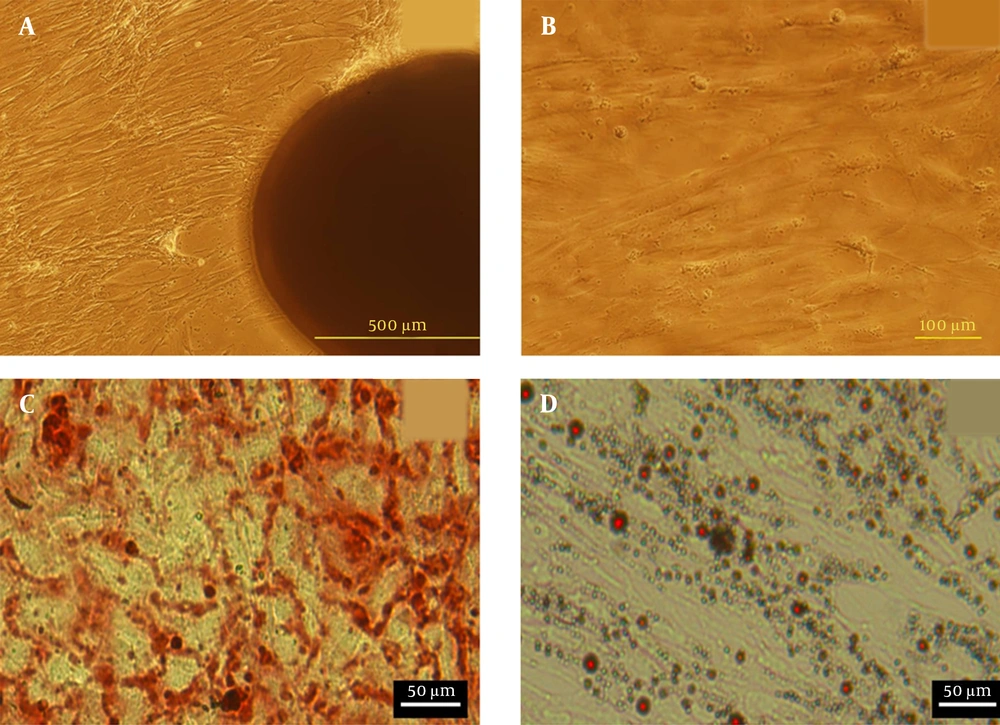
The morphology of hUC-MSCs was observed by light microscope (Figure 3A and B). Approximately 12 days after cultivation, the hUC-MSCs migrated out from Wharton’s jelly pieces. After this time, tissue fragments were removed from flasks, and cells were cultured up to three passages. Microscopic studies exhibited fibroblast-like morphology with a homogeneous population of MSCs. This monolayer of MSCs can then be used for cultivation over the scaffold. The flow cytometry analysis of cultured hUC-MSCs at passage 3 showed that CD29, CD90, and CD105 surface markers were expressed at high levels (88.12 ± 2.3%, 89.85 ± 0.27%, and 95.73 ± 0.31%, respectively). Also, the results demonstrated that the expression of hematopoietic lineage markers CD34 and CD45 were negative (3.21 ± 0.01% and 2.93 ± 0.13%, respectively). In addition, hUC-MSCs in the osteogenic medium showed mineralization of calcium was detected by Alizarin Red S staining. Also, adipogenic differentiation of cells were confirmed using Oil Red O staining (Figure 3C).
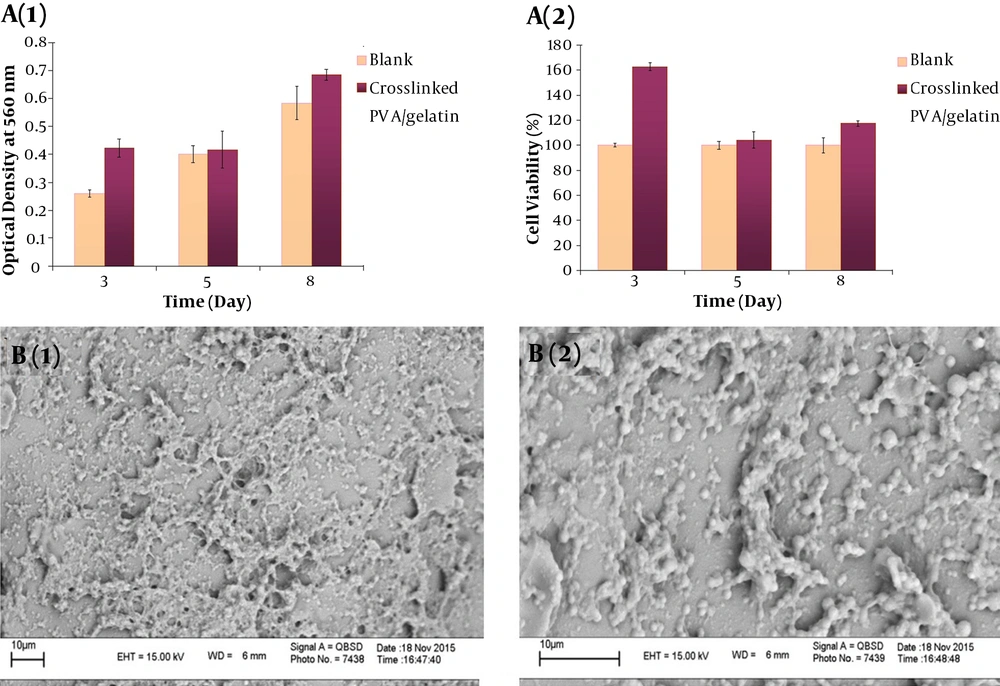
4.6. MTT Assay and Cell Morphology
MTT assay was used to study the biocompatibility of the crosslinked PVA/GE scaffold with the hUC-MSCs after 3, 5, and 8 days of culture (Figure 4A). The average viability difference of the cells on crosslinked scaffolds was higher than that of the control group at the three evaluation time points and were all statistically significant (P < 0.05). It was 15.9%, 1.6%, and 10.1%, after 3, 5, and 8 days of culture, respectively. Also, Wharton’s jelly hUC-MSC morphology and distribution over the crosslinked PVA/GE scaffold were observed by SEM at various magnifications. hUC-MSCs were adhered and spread well over the surface of the scaffold and covered the pores with high integration (Figure 4B). After 48 hours of culture on the crosslinked PVA/GE scaffold, the cells formed a homogeneous distribution.
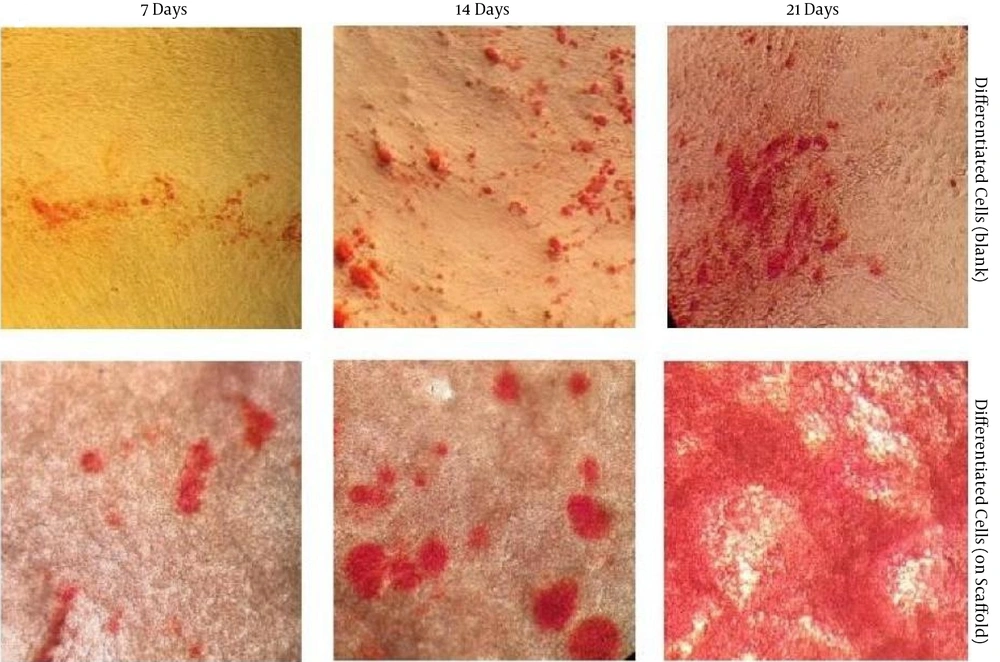
4.7. Alizarin Red Staining and SEM Images of Differentiated Cells
Under osteogenic conditions, the differentiation potential of the hUC-MSCs cultured over the crosslinked scaffold for 21 days was assessed. Calcium deposition was detected by the appearance of a red-orange color that was indicative of osteogenic differentiation (24). The amount of calcium deposition was higher in the 3D cultures compared to the control group, and on day 21, it reached its maximum amount compared to all other days of the experiment (Figure 5).
5. Discussion
Gelatin as a natural polymer was used to fabricate nanofibrous scaffolds due to its high cell affinity, but low processability and weak physical resistance. Meanwhile, synthetic polymers, such as PVA, have sufficient physical strength. However, it has a low tendency to adhere to cells due to the lack of cell recognition sites. Hence, in some previous studies, the preparation and utilization of PVA/GE scaffolds have been reported. Ceylan et al. (25) prepared PVA/GE cryogels by chemical and physical crosslinking of PVA and gelatin mixture. They demonstrated that the physically crosslinked cryogels degraded faster than the chemically cross-linked ones. However, the scaffolds crosslinked physically showed better biocompatibility. Besides, Linh et al. (26) fabricated PVA, GE, and PVA/GE nanofibers by electrospinning. They investigated the effect of process parameters (i.e., the concentration of GE in PVA/GE blends, electrical field, and tip-to-collector distance) on the chemical, morphological, and mechanical properties of the prepared scaffolds. Also, Linh and Lee (27) produced electrospun nanofibrous PVA/GE that was physically crosslinked by methanol treatment. They seeded MG-63 cells on the manufactured scaffold and showed that osteoblasts could attach and proliferate on the nanofibrous PVA/GE.
The aim of this study was to fabricate a biocompatible PVA/GE nanofibrous scaffold for osteogenic differentiation of MSCs. For this purpose, we fabricated a PVA/GE scaffold to obtain both mechanical strength through PVA support and biological properties through gelatin. The water-soluble nature of PVA and gelatin makes them insufficient for applications in aqueous media, such as biological systems. In this regard, we performed crosslinking to improve this property of the scaffolds and make them water-resistant and useful for tissue engineering applications. One of the most influential and broadly used chemical crosslinking agents is glutaraldehyde (GA). GA can crosslink polymers like gelatin and PVA that have hydroxyl and amine groups. In the crosslinking step, a Schiff’s base reaction occurs through aldehyde groups of GA and amine groups of gelatin, as can be proved by FTIR and TGA. The porosity percentage of the crosslinked PVA/GE nanofibers was within the appropriate range for cellular activity and proliferation, which indicates its promising potential for use as a biomaterial in bone tissue engineering applications.
The surface wettability of nanofibrous scaffolds is a crucial property that can affect cell behavior. As seen in Appendix 2 in Supplementary File, the scaffold crosslinked with GA/methanol indicated notably higher contact angles compared to the non-crosslinked scaffold. This result indicated that the imperfect hydrophobic nature of non-crosslinked PVA/GE that causes problems for use in aqueous media was improved by crosslinking. Also, the obtained results demonstrated that swelling property, degradation behavior, and pH changes of the crosslinked scaffold were appropriate for the tissue engineering application. The hydrophobic properties of the scaffold and dissolution rate of oligomers affected the swollen behavior of scaffold, which was related to the balance between the degradation rate of oligomers in the solution and the water uptake by nanofibers (28). From the MTT results, it became clear that the non-toxic property of the crosslinked scaffold provides good hUC-MSCs attachment. Due to the presence of numerous polar groups in PVA and gelatin structures, they are known as hydrophilic polymers. Therefore, scaffolds containing PVA/GE can be destroyed and dissolved easily in aqueous solutions (such as PBS). However, crosslinking leads to the formation of a 3D network of polymers, which can provide a stable environment for the adhesion and proliferation of seeded cells. SEM images indicated that uniform structure of electrospun scaffold provided a suitable environment for cell proliferation and differentiation. As shown by SEM images, the introduced PVA/GE nanofibrous electrospun scaffold had excellent performance for supporting the osteogenic differentiation of hUC-MSCs into osteoblast-like cells.
5.1. Conclusions
A PVA/GE nanocomposite scaffold was successfully prepared by the electrospinning technique. Chemical crosslinking by GA and GA/methanol immersion was utilized to change scaffold solubility. This treatment influenced the morphology and solubility of the scaffold. Assay results revealed that scaffolds have an excellent swelling rate, degradation behavior, and pH changes. The in vitro cell culture studies using Wharton’s jelly-derived MSCs showed that nanofibrous scaffolds were biocompatible, and cells seeded on the scaffolds adhered to and proliferated on pore walls. Also, under osteogenic conditions, MSCs could be differentiated into osteoblast cells. These results show the potential application of PVA/GE nanocomposites in bone tissue engineering.