1. Introduction
Stress involves two-way communication between the brain and the cardiovascular, immune, and other systems via neural and endocrine mechanisms. A hallmark of the stress response is the activation of the autonomic nervous system and hypothalamic-pituitary-adrenal (HPA) axis, and the “fight-or-flight” response is the classical way of envisioning the behavioral and physiological response to a threat from a dangerous situation, be it a predator, a mugger, an accident, or natural disaster (1). Many mood disorders, including depression and anxiety, which are widespread psychiatric neuropsychiatric conditions, are also associated with stress (2). Hence, not surprisingly, exposure to chronic stress can lead to a broad variety of adverse physiological and psychological effects (3). Significant limitations of the current treatments (i.e., a high number of nonresponsive patients, high rate of relapse) is suggestive of the scientists’ failure to gain sufficient scientific knowledge of these devastating illnesses as well as to develop efficacious therapeutic strategies (4). Various pharmacological and non-pharmacological methods (e.g., physical activity) have been developed and recommended for treating anxiety and depression (3). However, the organism needs the normal stress hormone response to survive such situations, and inadequate or excessive adrenocortical and autonomic function is deleterious for health and survival (1). Therefore, some of the most important challenges when dealing with stress are the amount, duration, and intensity of it as well as how to recover from it.
On the other hand, HIF-1α functions as a master regulator for the expression of genes involved in the hypoxia response of most mammalian cells (5, 6). In fact, HIF-1α initiates transcription of various hypoxia-adaptive genes, such as angiogenesis, glycolysis, and erythropoiesis, after the formation of a heterodimer with HIF-1β. Vascular endothelial growth factor (VEGF) along with it, is the most potent endothelial-specific mitogen, which recruits endothelial cells into hypoxic foci and avascular areas and stimulates their proliferation (7). Nevertheless, it is clearly expressed in various tissues such as the brain, kidney, liver, heart, and skeletal muscle in mice kept in a normoxic condition (21% O2), whereas it is undetectable in the lungs (8). These findings indicate that there is a clear PO2 gradient between the lungs and other tissues, and that with the exception of relatively well-oxygenated lungs, low PO2 and HIF1α play physiologically essential roles in the homeostasis of various tissues (7).
Evidence accumulated during the past seven years has assigned HIF-1α a critical role in mediating cardioprotection (9). The role of HIF-1 in mediating cardioprotection was first recognized by Cai et al. in 2003 (10). Intermittent systemic hypoxia occurs in many common physiological and pathophysiological conditions in human life, which could be caused by environmental factors (e.g., high altitude) or by various cardiopulmonary disorders (e.g., heart failure, chronic obstructive pulmonary disease, and sleep apnea) and hematological diseases (e.g., anemia) (11). In fact, intermittent exposures to hypoxia are much more frequent than chronic exposure to hypoxia (9).
Interestingly, a few well-controlled protocols of intermittent hypoxia can induce protective effects against myocardial infarction in rodents via a signaling mechanism that depends on inducible nitric oxide synthase (iNOS) (11-13). Since the iNOS gene has the hypoxia responsive elements (HRE) in its promoter region, and HIF-1 is essential for the hypoxic regulation of iNOS gene expression in cardiomyocytes (14). It is logical to speculate a role for HIF-1 in intermittent hypoxia-induced cardioprotection. This concept was experimentally introduced by Cai et al., who demonstrated that the cardioprotective effect of intermittent hypoxia depended on HIF-1α gene dosage in wild-type mice, indicated by better left ventricular contractile function and significantly smaller infarct size following ischemia-reperfusion in the animals pretreated with intermittent hypoxia (10).
Hypoxia caused by exercise conditions may alter HIF-1α expression in cardiac cells (15). The study on acute exercise has shown that several components of the HIF-1 pathway, involving VEGF and erythropoietin (EPO) , are activated in response to acute changes in oxygen demand in human skeletal muscle (16), suggesting that oxygen-sensitive pathways could facilitate the adaptation to physical activity by increasing capillary growth. Sylviana et al., examining the effect of force swimming exercise on the expression of PGC-1α and HIF-1α gene expression in cardiac muscle of mice found that the ST group increased the expression of PGC-1α but decreased the expression of HIF-1α in mice cardiac muscle in response to chronic hypoxia condition (15). Furthermore, the effects of endurance training on the activity of the HIF pathway in human skeletal muscle appear to be higher than those under resting conditions, indicating that combining exercise training may improve some aspects of muscle O2 transport and/or metabolism. On the other hand, increased levels of reactive oxygen species (ROS) due to physical exercise induce the expression of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), which regulates mitochondrial biogenesis in multiple cell types, resulting in increases in VEGF expression and subsequent angiogenesis, and strongly suggesting HIF-1α-independent regulation of VEGF and angiogenesis. Taking into account the relationship among exercise, HIF-1α pathway, including VEGF, PGC-1α, and ROS (7), therefore, the present study aimed to examine the effect of swimming training on HIF-1α and VEGF levels in heart tissue of rats exposed to chronic stress.
2. Methods
A total of 30 male Wistar rats (age: 10 - 12 weeks, weight: 220 ± 20 g) were obtained from the Animal Centre of Medical Sciences (Shahid Beheshti University). The environment was maintained in a dark light cycle (12-h light cycle and 12 hours of dark cycle), humidity 40 - 50%, temperature of 22°C, and complete state of silence without any noise pollution and stressors. Rats were fed a pellet rodent diet ad libitum and had free access to water. To avoid the possible effect of rats with anxiety on healthy rats, they were housed in separated cages and places. The laboratory and training environments were washed and cleaned on a daily basis (17). All procedures implemented to study the animals were in accordance with the National Institutes of Health guide for the care and use of laboratory animals (18). The rats were randomly divided into five equal groups of six rats, including as follow: (1) animals performing no exercise and receiving no stress (Con); (2) animals exposed to chronic stress followed by performing swimming training (CS + ST); (3) animals exposed to chronic stress (CS); (4) animals receiving swimming training (ST); (5) animals exposed to chronic stress followed by a period of recovery time with neither training nor exposure to stress (CS-time). The open field test (OFT) was performed 48 h after administrating the treatments.
2.1. Swimming Training and Chronic Mild Stress (CMS) Protocols
Rats in the training groups performed 60 min of swimming per day, for five days a week, lasting for four weeks (19). All training sessions were performed under red light (as it causes the least amount of stress) at 6 pm, which is the best training time in the mice’s normal activity rhythm (20). To alleviate stress without promoting adaptation to exercise, all rats were adapted to water prior to initiating the experiment. Rats were exposed to mild chronic stress for 21 days, which included 2 h of paired caging, 18 h of free access to food followed by 1.5 h of restricted access to food (0.2 g pellet), 18 h of water deprivation followed by 1.5 h of empty bottle exposure, 21 h of the wet cage (300 mL of water added per 100 g of bedding), 36 h of continuous lighting and 3 h of 45° cage tilting. This stress program was pursued for four weeks (21).
2.2. Open Field Test
Locomotor activity was measured in an apparatus comprising a wooden platform enclosed by four white wooden walls (100 cm, 100 cm, 50 cm). The floor was divided by red lines into 25 equal squares (20 cm, 20 cm), and a central square was drawn using a black marker. The open field was placed inside a light- and sound-attenuated room. Rats were routinely tested during the first half of the dark phase of their light/dark cycle. The animals in three experimental groups were given a single i.p. dose of citicoline containing 50, 100, or 150 mg/kg of citicoline 30 min before starting the test. Those animals in two control groups were treated with diazepam at a dose of 2 mg/kg or with normal saline 30 min before starting the experiments. The test was performed following previously described procedures. Briefly, each rat was placed in a corner square of the open field apparatus, and its behavior was recorded for 5 min using a camera (Panasonic, Japan) placed above the apparatus (22).
2.3. Immunohistochemistry for Detection of HIF-1α Expression
Briefly, formalin-fixed tissue sections (3 μm thick) were deparaffinized in xylene and rehydrated through graded concentrations of ethanol. Antigen retrieval was then performed undergoing microwave treatment (3 cycles of 5 min. each). After incubation with the primary antibodies, positivity was revealed by using a commercially available immunodetection system followed by Mayer’s hematoxylin counterstaining for 3 min. Slides were then rinsed with ammonia water, washed in tap water, and covered using Glycergel. Positive controls (according to the manufacturer’s protocol) were included in the study. Sections stained with the same protocol through omitting the primary antibodies were used as negative controls (antibody: Anti-HIF1α, Cat No. GTX127309, rabbit, Thermo scientific, GENETEX, USA; Antigen retrieval: MW [MW (350 W, three times for 5 min. each) in 0.01 M citrate buffer, pH 6]; Dilution in TBS: 1/20; Incubation: Overnight).
2.4. Western Blotting
The tissues were lysed in lysis buffer (20 mM Tris, 150 mM NaCl, 0.25% NP-40, 1 mM phenylmethylsulfonylfluoride, and 1 × protease inhibitors). Fifty micrograms of protein were analyzed using (4 - 16%) gradient SDS-PAGE under denaturing conditions and electrotransferred to nitrocellulose membranes (Sigma). Nonspecific protein binding was blocked by incubating the membranes with blocking solution (PBS and 5% non-fat dried milk) for 60 min, at room temperature. Polyclonal antibodies specified (VEGF primary antibody, Cat No. GTX102643, GENETEX, USA) for VEGF (1: 250 in PBS containing 5% non-fat dried milk) were applied to the membrane and incubated overnight at 4°C. After washing the membranes with TBST for 5 min, they were incubated with anti-horse/rabbit or anti-goat/rabbit immunoglobulin G (IgG)-horse radish peroxidase (HRP) (Dingguo Changsheng Biotech, Beijing, China) for 1 h. The membranes were all washed with TBST four times for 5 min. Bound antibody chemiluminescence was detected using chemiluminescence kits (Thermo Scientific, Germany). The optical density was determined using a scanning densitometer and analyzed using Quantity One software (Bio-Rad). The b-Actin was used as the internal control.
2.5. Statistics
All statistics were performed using SPSS 20.0 software, and the results were presented as the mean±standard error of the mean (mean ± SEM). To analyze the data, Kolmogorov-Smirnov, One-way ANOVA, and Tukey’s post hoc tests were used, and P ≤ 0.05 was considered statistically significant.
3. Results
3.1. Anxiety-Like Behaviors (Confirmation of Stress Induction)
Anxiety-like behaviors were significantly different among the five group conditions (P < 0.001). Post-hoc analyses showed that compared to the CON, the total distance [F (4, 25) = 78.77, P < 0.001], average speed [F (4, 25) = 187.06, P < 0.001], and wall time [F (4, 25) = 69.85, P < 0.001] were significantly shorter in the CS (P < 0.001) and in the CS-time condition (P < 0.001). Furthermore, post-hoc analyses demonstrated that compared to the CON, corner time [F (4, 25) = 125.82, P < 0.001] and number of excrements [F (4, 25) = 11.38, P < 0.001] were significantly longer in the CS (P < 0.001) and in the CS-time condition (P < 0.001).
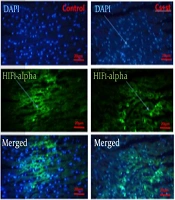
3.2. Detecting HIF-1α Protein by Immunohistochemistry Technique in Heart Tissue of Rats Subjected to CMS in Response to Swimming Training
This study aimed to investigate the modulation of HIF-1α protein expression in heart tissue of rats subjected to chronic mild stress (CMS) by western blot (Figure 1). Our results revealed that HIF-1α levels were significantly different among the five group conditions [F (4, 25) = 20.8, P < 0.0001]. Tukey's multiple comparison test showed that chronic mild stress significantly decreased the HIF-1α expression in heart tissue (in CS and CS-time groups) (P < 0.05). Moreover, the result determined that swimming training significantly increased the level of HIF-1α expression in heart tissue (in ST and CS + ST groups) (P < 0.05) (Figure 1). Although swimming training after a period of four weeks of CMS (in the CS + ST group) increased HIF-1α levels, these increases were smaller than those observed in the ST and control groups (P < 0.05). Even though swimming training increased HIF-1α levels in the CS + ST group, no significant difference was detected between the control and CS + ST groups in this regard (Figure 1).
Immunohistochemistry analysis of HIF-1α protein levels in Con, CS + ST, ST, CS and CS + R groups [data are expressed as mean ± SD; Non-identical letters are significant to each other; *P < 0.05; CS + ST, chronic mild stress; CS, chronic mild stress; ST, swimming training; CS time, chronic mild stress-time (or recovery)].
3.3. Investigating the Changes VEGF Protein Expression by Western Blotting in Heart Tissue of Rats Subjected to CMS in Response to Swimming Training
According to the One-way ANOVA test, significant differences were detected among five groups of rats in terms of the levels of VEGF [F (4, 25) = 163.2, P < 0.0001] in the heart tissue (Figure 2). Tukey's multiple comparison test showed that chronic mild stress significantly downregulated the VEGF expression in heart tissue in CS and CS-time groups, whereas swimming training has significantly increased the level of it in ST and CS + ST groups (P < 0.05) (Figure 2). Although swimming training increased VEGF levels in the CS + ST group after four weeks of CMS, these increases were smaller than those observed in the ST and control groups (P < 0.05) (Figure 2).
Western blot analysis of VEGF protein levels in Con, CS + ST, ST, CS, and CS + R groups [data are expressed as mean ± SD; Non-identical letters are significant to each other; *P < 0.05; CS + ST, chronic mild stress; CS, chronic mild stress; ST, swimming training; CS time, chronic mild stress-time (or recovery)].
4. Discussion
Chronic stress has been regarded as one of the major physical and mental health issues (4). Chronic stress-induced anxiety disorders are highly-prevalent and modern social diseases in which oxidative stress plays an important role. Developing an effective treatment for anxiety disorders specifically requires identifying the underlying mechanisms governing these disorders (23). In this study, the effect of swimming training on HIF-1α and VEGF levels in heart tissue of rats subjected to CMS was examined. Our study findings revealed that CMS may have considerably decreased HIF-1α levels in the CS-time and CS groups; however, swimming training increased HIF-1α levels in the CS+ST and ST groups, which were consistent with the results from some other studies (6, 24). In addition, swimming training significantly increased the HIF-1α levels in ST group in compared to the CS + ST and control conditions. HIF1α is a transcription factor regulated by cellular oxygen concentration that initiates gene regulation of vascular development, redox homeostasis, and cell cycle control (25). In fact, HIF-1α functions as a master regulator for the expression of genes involved in the hypoxia response of most mammalian cells (5), and contributes to important adaptive mechanisms that occur when oxygen and ROS homeostasis become unbalanced. It has been shown that preconditioning it by exposure to a stressor prior to a hypoxic event reduces the damage that would otherwise occur (25). Furthermore, there has been growing evidence that HIF-1α protein stability is regulated by oxygen-independent mechanisms (24). For example, acute exercise is accompanied by the regional and systemic reduced partial pressure of oxygen as well as acidosis, oxidative stress, and heat, all of which are stimulatory factors of HIF-1α (26). The reduction in oxygen availability due to exercise requires the changes in cells’ metabolism to adapt to the catabolic and anabolic reactions that rely on the availability of ATP normally supplied by mitochondrial oxidative phosphorylation (OXPHOS) (27). HIF-1α signaling reduces cell dependence on oxygenated energy products by downregulating OXPHOS (28). A study found that after exercise training, the slow isoform I of both heavy and light myosin subunits increased and the fast isoform IIa decreased, suggesting chronic hypoxia results in a fast-to-slow muscle fiber transition, which could lead to a faster activation of mitochondrial oxidative metabolism (29). In fact, both hypoxia and exercise are able to increase HIF-1α accumulation (6). A recent study has shown that the skeletal muscle HIF-1α protein content is 120% higher after hypoxia exposure, and is further induced by exercise (30). Compared to resting in normoxia, exercise in hypoxia raises the HIF-1α protein expression approximately 2.5-fold (30). When the oxygen supply is insufficient, HIF-1α target genes improve oxygen transport by EPO-mediated erythropoiesis and VEGF-induced angiogenesis mechanisms and mediate skeletal muscle adaptions to endurance training through optimized glucose transport and glycolytic enzyme activity. Finally, exercise could increase PGC-1α mRNA expression (31), which induces mitochondrial biogenesis.
Moreover, reactive oxygen species (ROS) are produced from mitochondrial respiration by leakage of electrons from the electron transport chain to oxygen. This process is accelerated during exercise as a result of the increased mitochondrial activity (32). ROS stabilize HIF-1α possibly by interfering with the activity of Fe2+-dependent proline hydroxylases. ROS may also activate the MAPK or PI-3K/Akt pathway, which enhances the transcriptional activity of HIF-1α through its phosphorylation. Finally, mechanical stress may increase ROS production that can influence HIF-1α activation (33). In normoxia/rest, moreover, low HIF-1α levels were found, lending support to the idea that HIF-1α is hydroxylated by a prolyl hydroxylase recognized by von Hippel-Lindau tumor suppressor protein (VHL) as well as a ubiquitin-protein ligase, and targeted for degradation by the proteasome. However, the exercise increases oxygen consumption and reduces oxygen tension to levels that inhibit prolyl hydroxylase, resulting in accumulation of the HIF-1α protein and translocation into the nucleus. In the nucleus, HIF-1α and ARNT (HIF-1β) dimerized and activated target genes such as VEGF and erythropoietin (EPO). In addition, no further activation of HIF-1 was observed when oxygen delivery to the exercising muscle was reduced (16).
On the other hand, our findings indicated that swimming training increased VEGF levels in the CS + ST and ST groups, though CMS was able to considerably decrease their levels in the CS + CS-time and CS groups. In addition, swimming training significantly increased the VEGF levels in ST group in compared to the CS + ST group; however, there was no significant difference between the given group and the control group in this regard. Previous studies on exercise have shown that several components of the HIF-1 pathway, involving VEGF and erythropoietin, are activated in response to acute changes in oxygen demand in human skeletal muscle, suggesting that oxygen-sensitive pathways could be relevant factors contributing to the adaptation to physical activity by increasing capillary growth. On the other hand, increased levels of reactive oxygen species (ROS) due to physical exercise induce the expression of PGC-1α, which regulates mitochondrial biogenesis in multiple cell types, resulting in increases in VEGF expression and subsequent angiogenesis and strongly suggesting the HIF-1α-independent regulation of VEGF and angiogenesis (7). In fact, Exercise induces a range of adaptations, including upregulation of angiogenesis that, in turn, contributes to exercise adaptations (34). Several studies have investigated the changes associated with angiogenesis after exercise and found that the VEGF mRNA expression increases in rat skeletal muscle eight weeks after moderate-intensity incremental treadmill exercise (35). Some others studies have also demonstrated that blood VEGF and ANGP-1 are significantly increased after eight weeks of resistance training and that moderate-intensity resistance training might lead to higher angiogenesis compared to high-intensity resistance training (36). The interstitial content of VEGF protein has been shown to increase after a session of moderate-intensity exercise (60 min of exercise at ~ 64% of VO2max) and a session of SIE (24 × 1 min, at 117% of VO2max, separated by 1.5 min of passive rest); however, the increase caused by moderate-intensity exercise is greater than that produced by SIE (37). In human skeletal muscle, endurance exercise has been discovered to decrease the protein content of anti-angiogenic regulators, together with an increased capillarity in the muscle (38).
4.1. Conclusions
It was concluded that chronic mild stress had the potential to decrease HIF-1α and VEGF levels considerably, while swimming training had the capacity to increase HIF-1α and VEGF levels (in ST and CS + ST groups). Seemingly, HIF-1α influenced the training-induced VEGF expression or, alternatively, HIF-1α and VEGF expressions were co-regulated at the transcriptional level in heart tissue. Overall, the cumulative effects of swimming training on the levels of hypoxic factors may have represented the underlying kinetic basis for the cellular adaptations associated with modulating the negative effects of stress.
![Immunohistochemistry analysis of HIF-1α protein levels in Con, CS + ST, ST, CS and CS + R groups [data are expressed as mean ± SD; Non-identical letters are significant to each other; *P < 0.05; CS + ST, chronic mild stress; CS, chronic mild stress; ST, swimming training; CS time, chronic mild stress-time (or recovery)]. Immunohistochemistry analysis of HIF-1α protein levels in Con, CS + ST, ST, CS and CS + R groups [data are expressed as mean ± SD; Non-identical letters are significant to each other; *P < 0.05; CS + ST, chronic mild stress; CS, chronic mild stress; ST, swimming training; CS time, chronic mild stress-time (or recovery)].](https://services.brieflands.com/cdn/serve/3170b/fd10f7538b2d04ade10bd4a47b28703e48ac02f4/gct-116825-i001-F1-preview.webp)
![Western blot analysis of VEGF protein levels in Con, CS + ST, ST, CS, and CS + R groups [data are expressed as mean ± SD; Non-identical letters are significant to each other; *P < 0.05; CS + ST, chronic mild stress; CS, chronic mild stress; ST, swimming training; CS time, chronic mild stress-time (or recovery)]. Western blot analysis of VEGF protein levels in Con, CS + ST, ST, CS, and CS + R groups [data are expressed as mean ± SD; Non-identical letters are significant to each other; *P < 0.05; CS + ST, chronic mild stress; CS, chronic mild stress; ST, swimming training; CS time, chronic mild stress-time (or recovery)].](https://services.brieflands.com/cdn/serve/3170b/92b6ab6c0485343933edcb71797200f8c07fdff5/gct-116825-i002-F2-preview.webp)