1. Background
Skin cancer is one of the most common cancers and affects men more than women (1). Studies have shown that skin cancer is the most common type of cancer in the Middle East (2). The origin of skin tumors can be basal layer cells [basal cell carcinoma (BCC)], squamous cells [squamous cell carcinoma (SCC)], melanocyte cells (malignant melanoma), immune cells (lymphoma, etc.), skin appendages, etc. (3). The incidence of skin cancers has increased in recent years (4). According to studies in the Netherlands and the United Kingdom, this could be due to increased daily outdoor activities without adequate clothing, increased seaside travel, prolonged sun exposure, and ozone depletion (5). Sensitivity of the skin to light is one of the side effects of some drugs. This is due to the ability of drugs or their metabolites to absorb energy from sunlight (6) and can significantly increase the risk of non-melanoma skin cancer (NMSC). Optical sensitivity can be achieved through an exciting chromophore (optical sensitivity type I) and/or the formation of single oxygen (type II) (7). Light-sensitive activated oxygen species can damage nucleic acids and their precursors and the membrane’s proteins and lipids (8). Fluoroquinolone antibiotics have been identified as a light sensitizer with a wide range of adverse skin reactions (9). They have photo-carcinogenic effects in mice (10) and increase the risk of pre-malignant skin lesions in patients (11). Fluoroquinolone is structurally based on nalidixic acid, which has been replaced by fluorine C6. Fluoroquinolones inhibit DNA gyrase and topoisomerase IV enzymes, which are essential for bacterial proliferation (12). They are also ultraviolet (UV) chromophores with absorption peaks at 280 - 315 nm (UVB) and 315 - 400 nm (UVA). These drugs can form cyclobutane thymine dimers (T<>T CPDs) to break DNA strands and the oxidized bases by both type I and II reactions (13, 14). Ciprofloxacin is the most common fluoroquinolone and is more associated with skin side effects than other fluoroquinolones (8). Since the advent of the first lasers, their use in medicine has expanded dramatically (15). By careful selection of parameters, lasers can be used to target tumor components. Low-power laser (LPL) can be an alternative treatment option that minimizes the side effects of current treatments used to treat NMSC (16).
2. Objectives
The aim of this study was to investigate the effect of ciprofloxacin and LPL on apoptosis, cell viability, and reactive oxygen species (ROS) Levels in the A375 cell lines.
3. Methods
This research was conducted in the Cellular and Molecular Laboratory of Islamic Azad University, Tehran branch (ID: 101290532353935) in 2020.
3.1. Cell Culture
The A375 cancer cells were purchased from the Cell Bank of the Iranian Biological Resource Centre, Tehran, Iran. The cells were cultured in 25 mL plastic flasks containing DMEM (DENAzist Asia, Iran), FBS 10% (DENAzist Asia, Iran), and 10µg/mL penicillin-streptomycin solution. The cells were incubated in a CO2 incubator (MEMMERT, IN55) at 37°C with 5% CO2 and humidity of 80%. The culture medium was replaced with fresh medium three times a week. Cell counting was performed using a hemocytometer (HGB, Germany).
3.2. MTT Assay
MTT assay was used to measure the percentage of cell viability (17). Briefly, 3 × 104 cells were cultured in a 96-well plate and incubated overnight at 37°C with 5% CO2 and humidity of 80%. Then, the cells were treated with different concentrations of ciprofloxacin (5, 25, 50, 75, and 100 μg/mL) for 24, 48, and 72 hours. The test group was irradiated with LPL (2 J/cm-2) for 30 s. The negative control cells were not treated with laser beams and ciprofloxacin, but the positive control cells were only treated with ciprofloxacin. Then, 5 µl of MTT stock solution (5 mg/mL) was added to each well and incubated for 1 h at 37°C. When the purple colored precipitates were observed under an inverted microscope (ZEISS, Germany), the media was carefully removed, and 100 µL of dimethyl sulfoxide (DMSO) was added to each well, and the plate was incubated at room temperature for 2 h. Finally, the absorbance was measured at 570 nm using a microplate reader (Bio-Rad, USA). The assay was repeated in triplicate for each concentration, and the cell viability was determined using the following formula:
3.3. Microscopic Studies
The A375 cells were evaluated in three different groups, (1) treatment with ciprofloxacin (1 mg/mL); (2) treatment with ciprofloxacin (1 mg/mL), and LPL (660 nm; power density: 30 mW cm−2 for 30 s); and (3) treatment with LPL (660 nm; power density: 30 mW cm−2 for 30 s). Then, using a reverse microscope, microscopic images were obtained from the samples.
3.4. Evaluation of Levels of Reactive Oxygen Species (ROS) by Flow Cytometry
The levels of ROS in the treated cells were measured by the flow cytometry method. Accordingly, 1 × 106 cells were cultured in 6-well plates, and then the cells were treated with ciprofloxacin (5, 25, 50, 75, and 100 μg/mL) for 24, 48, and 72 hours, followed by irradiating with LPL at 660 nm for 30 s. The cells were removed from the plate and incubated with 2 mM of DCFH2-DA for 45 min in the dark place. Then, the cells were washed with PBS and transferred to a flow cytometer for measuring the ROS levels. The data were analyzed by FlowJo (7.6.1) software (18).
3.5. Evaluation of Apoptosis by Flow Cytometry
To detect the apoptotic cells, the FITC Annexing V Apoptosis Detection Kit (BD Pharming™, 556547) was used. The A375 cells were subjected to photodynamic therapy (PDT) and washed twice with 200 μL of PBS and detached from the plastic flasks using trypsin-EDTA solution (Gibco Invitrogen, USA). Then, 1 × 106 cells/mL were resuspended in 200 μL binding buffer (1x), and 5 μL of FITC Annexing V and 5 μL of propidium iodide (PI) were added to the cell suspension. Stained cell samples were incubated for 10 min at room temperature, and 200μL of binding buffer (1x) was again added to cell suspension prior to analysis with a flow cytometer. The data were analyzed by FlowJo (7.6.1) software (19).
3.6. Statistical Analysis
All tests were performed in triplicate, and the results were reported as mean ± SD. The obtained data were analyzed using GraphPad Prism V. 8 (La Jolla, CA, USA) and SPSS 26 software by two-way analysis of variance (ANOVA). A P < 0.05 was considered significant.
4. Results
4.1. Microscopic Examination
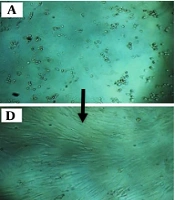
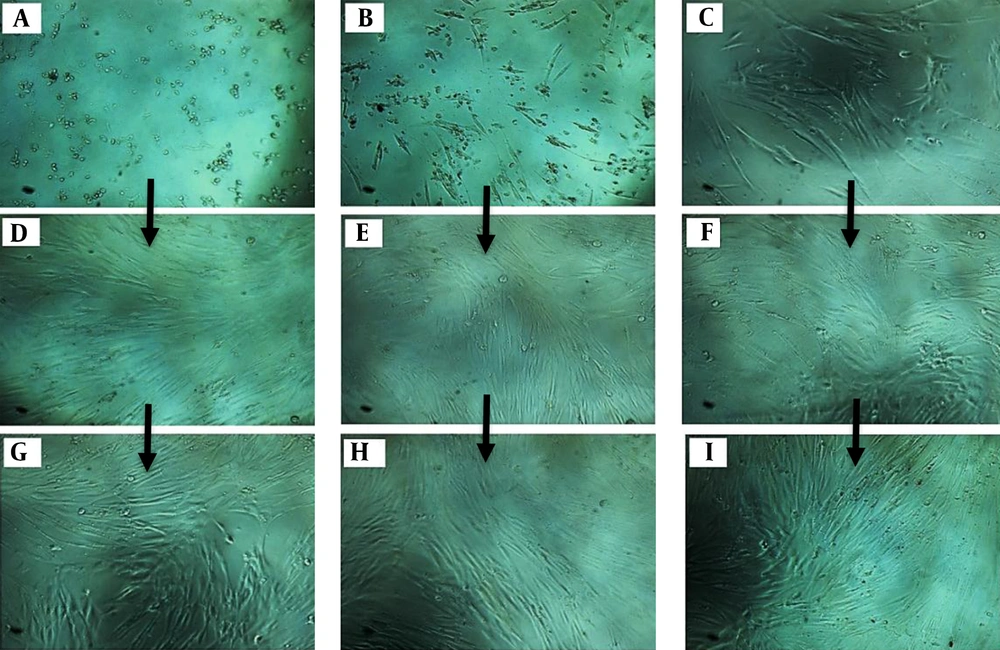
According to Figure 1, the cell proliferation rate was evaluated at 24, 48, and 72 hours. The cell proliferation in the cells treated with ciprofloxacin and LPL showed a significant decrease compared to other groups (B, E, and H). Groups 1 [ciprofloxacin (1 mg/mL) for 72 hours] (Figure 1A, D and G) and 3 (Figure 1C, F and I) showed the same results. These results indicated the positive effect of concomitant use of ciprofloxacin and LPL to reduce the number of cancer cells.
A, D and G, treatment of A375 cancer cells with ciprofloxacin (1 mg/mL); B, E and H, treatment of A375 cancer cells with ciprofloxacin (1 mg/mL) + low-power laser (660 nm; power density: 30 mW cm-2 for 30 s); C, F, and I, treatment of A375 cancer cells with low-power laser (660 nm; power density: 30 mW cm-2 for 30 s).
4.2. MTT Assay
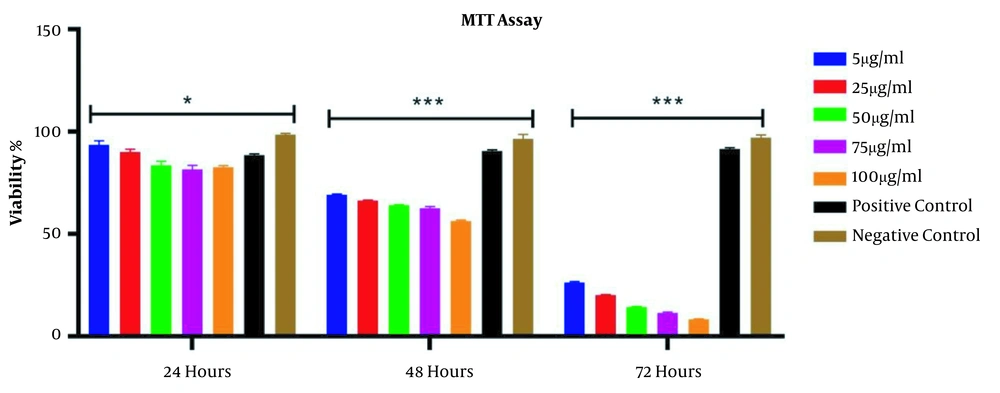
According to Figure 2, over time (24 and 72 hours after treatment), the percentage of cell viability decreased significantly in all groups. The results also showed that compared to the negative control group at 24, 48, and 72 hours, the percentage of cells viability after treatment with ciprofloxacin (5 to 100 μg/mL) and LPL significantly decreased (P < 0.001). This reduction was most noticeable at 72 hours caused by the synergistic effect of LPL and ciprofloxacin.
4.3. Evaluation of ROS Levels by Flow Cytometry
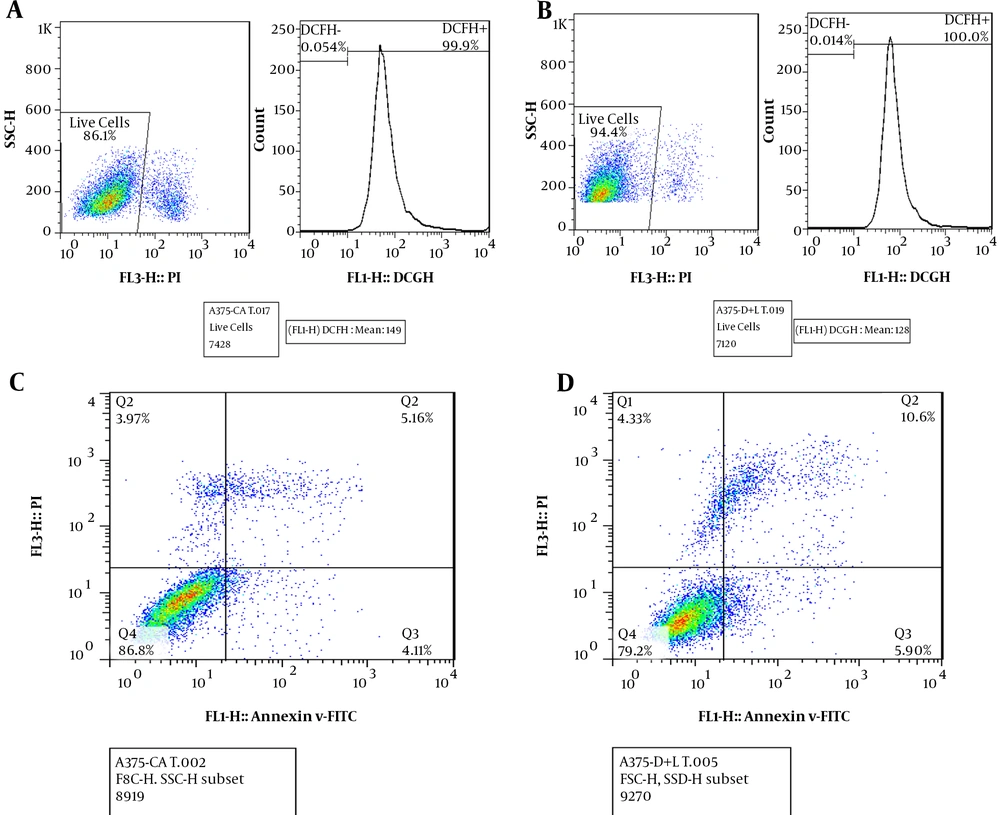
According to Figure 3, flow cytometry results showed that in A375 cells treated with ciprofloxacin (100 μg/mL) and LPL (Figure 3B), ROS levels decreased slightly (by 0.1%) compared to cells treated with ciprofloxacin without LPL radiation (Figure 3A). This result showed the effect of a combination of ciprofloxacin and LPL on the ROS levels of cancer cells.
A, measurement of ROS levels after treatment of A375 cells with ciprofloxacin (100 μg/mL); B, measurement of ROS levels after treatment of A375 cells with ciprofloxacin (100 μg/mL) and low-power laser (2 J/cm2, 30s); C, measurement of apoptosis after treatment of A375 cells with ciprofloxacin without laser irradiation; D, measurement of apoptosis after treatment of A375 cells with ciprofloxacin and low-power laser.
4.4. Evaluation of Apoptosis by Flow Cytometry
According to Figure 3, the results of flow cytometry showed that the percentage of apoptosis in A375 cells treated with ciprofloxacin and LPL (D) compared to A375 cells treated only with ciprofloxacin (C) increased by about 5%. Therefore, treatment with a combination of ciprofloxacin and LPL increased apoptosis in A375 cells.
5. Discussion
The results of the MTT assay showed that over time from 24 to 72 hours, the viability percentage of A375 cells decreased significantly. Also, 48 and 72 hours after treatment, the cell viability at concentrations of 5 to 100 μg/mL of ciprofloxacin and LPL significantly decreased (P < 0.001). The results of microscopic studies are consistent with the results of the MTT assay in this study. In the present study, the results of the MTT assay confirmed the results of morphological studies, and both tests showed that the treatment of A375 cells with a combination of ciprofloxacin and LPL increased cell death.
Various studies have shown that ciprofloxacin has a cytotoxic effect on skin cancer cells. For example, Beberok et al. (2018) showed that ciprofloxacin inhibited melanoma cells (COLO829) in the S phase of the cell cycle and induced apoptosis in these cells. The results of the MTT assay in this study showed that ciprofloxacin reduced the viability percentage in COLO829 cells (20). Aldaghi and Jalal in 2019 showed that ciprofloxacin has inhibitory and cytotoxic effects on A375 cells. They showed that ciprofloxacin has a cytotoxic effect and inhibits the growth of cancer cells by inhibiting DNA synthesis in the S phase of the cell cycle (21). In the present study, ciprofloxacin, through its cytotoxicity effects inhibited the growth and proliferation of A375 cancer cells. Swen et al. examined the effect of a low-power helium-neon laser on melanoma cells (cells A375 and A2058). They showed that LPL irradiation (2 J/cm2) increased the growth of A2058 cells but did not affect the growth of A375 cells (22). Their results contradict the results of the present study because we observed that LPL irradiation increased the apoptosis of A375 cells. It seems that the difference in the type of laser can affect the apoptosis of A375 cells. Also, ciprofloxacin in this study can have a synergistic effect and increase cell death and toxicity in A375 cells. In 2020, Khorsandi et al. studied the anti-cancer effect of LPL in combination with Gallic acid on A375 cells. They showed that treatment of A375 cells with Gallic acid and LPL increased the death rate in A375 cells (23). In this study, similar results were obtained with the simultaneous treatment of A375 cells with LPL and ciprofloxacin.
Flow cytometry results showed that ROS levels had a slight increase in A375 cells treated with ciprofloxacin and LPL compared to cells treated only with ciprofloxacin. Sunwoo et al. (2020) showed that the antibiotic ciprofloxacin increased ROS production and induced apoptosis in these cells by damaging the mitochondrial membrane and releasing cytochrome c (24). Beberok et al. (2018) showed that treatment of breast cancer cells (MDA-MB-231) with ciprofloxacin significantly reduced glutathione (GSH) levels in the cells, which is associated with increased ROS levels in the cell. The results of their study showed that treatment of cancer cells with ciprofloxacin increased ROS in these cells, which ultimately leads to mitochondrial damage and induces apoptosis in these cells (20). In the present study, treatment of A375 cells with ciprofloxacin and LPL increased ROS production, which is consistent with their results.
The results of flow cytometry showed that the number of apoptotic cells treated with ciprofloxacin and LPL increased by about 5% compared to A375 cells treated only with ciprofloxacin, which is consistent with other studies. For example, Wu et al. (2007) showed that LPL irradiation of human lung adenocarcinoma cells induced apoptosis in these cells and also changed the mitochondrial membrane potential, and activated the caspase-3 enzyme (25). Tian et al. studied the effect of LPL on apoptosis in human colorectal cancer (HT29) cells. They demonstrated that LPL inhibited cell migration and induced apoptosis in these cells (26).
In general, treatment of the A375 cell line with ciprofloxacin and LPL induced cell death and apoptosis and increased ROS levels. It can be concluded that the concomitant use of ciprofloxacin and LPL can increase cell death in the form of apoptosis in A375 cells, which can be effective in designing new methods to treat skin cancer.
5.1. Limitation and Suggestion
Due to an increase in the prevalence of different types of cancers, the need for new therapies is more than ever needed. The combination of LPL with other classes of antibiotics can be an effective way to offer new therapies in the treatment of various cancers.