1. Background
Continuous and indiscriminate use of chemical drugs has caused the critical phenomenon of resistance to microorganisms, reducing the effect of medicines. Due to the increased consumption of medicine and the trend in using new composites, this resistance has become stronger. Additionally, another disadvantage of using these drugs is their side effects, which can lead to diseases more dangerous than the original disease (1, 2).
Research on bacterial resistance to antitoxins first initiated in the 1960s and has gained momentum globally over the past 20 years. Therefore, the World Health Organization (WHO) and other health organizations have announced a battle against human microbial pathogens, particularly those acquired in hospitals. Because these bacteria are not resisted to different conventional treatments, medicinal plants have been used and evaluated for their therapeutic potential in different countries. These valuable biological resources are a repository of complex active molecules (3).
The study of medicinal plants to discover new treatments with fewer side effects and higher economic value has become particularly essential worldwide. According to published reports, more than 30% of herbal medicines are currently used in hospitals and clinics (4). Antibiotics are valuable drugs to treat infections; however, the overuse of these medicines will lead to microbial resistance. Therefore, scientists have prioritized experimentation on various components of medicinal herbs to identify new medicines of plant origin (5).
Iran has a long history in the field of traditional medicine and the use of medicinal plants for treating diseases. The rich flora of Iran and high knowledge of Iranians in the use of medicinal plants, the existence of reputable scientific centers in the cities of Isfahan, Shiraz, and Rey, and the existence of authoritative scientific sources such as Ibn Sina's book (Qanon) and famous scientists such as Abu Ali Sina and Razi, who have popularized herbal medicine among Iranians, and the interest of Iranians in medicinal plants, have doubled the need to pay attention to this field of science (3, 6). Numerous plant essential oils have been reported to have significant inhibitory effects against pathogenic microorganisms (7), which has led to a new wave of extensive global studies and the introduction of antibacterial effects of various plants in recent years (8).
So far, the antimicrobial properties of some medicinal plants have been evaluated against the bacterium Salmonella typhimurium. In addition, the antimicrobial properties of dill and coriander seed essential oils on Staphylococcus aureus, Escherichia coli, and S. typhimurium (9), antimicrobial properties of hydroalcoholic extracts of some medicinal plants on Listeria monocytogenes, S. typhimurium, and S. aureus (10), anti-cancer properties of Artima urmiana against S. typhimurium TA100 (11), anti-mutagenic properties of ethanolic and aqueous extracts of mangrove on the mutant Salmonlatifactive bacterium of Morium TA100 (12) have been established (13).
Salmonella typhimurium (causing gastrointestinal tract infection) is a Gram-negative, rod-shaped, capsule-free, non-acid fast bacterium that grows in most food environments and is able to ferment glucose and mannose. This microorganism is always the most common serological variety isolated from foodstuffs worldwide (14).
Salmonella typhimurium infections are common worldwide, especially in the developing countries, including Iran where antibiotics are used in many cases to treat them. The use of antibiotics to treat the disease can cause two crucial problems. First, it causes irreversible side effects, such as chloramphenicol, which can lead to aplastic anemia, and second, it can lead to drug resistance (15). Thus, medicinal plants are the best candidates for inhibiting antibiotic-resistant infectious bacteria, the advantages of which include low production costs, low side effects, and no environmental problems (16). As a result, in this study, different extracts of R. stricta were evaluated against S. typhimurium.
Rhazya stricta belongs to the Apoynaceae family and is a subfamily of the Rauwolfioideae family. R. stricta is known in Saudi Arabia as Esfand. R. stricta is an evergreen and small plant. This plant has been used as a medicine for treating diseases in Afghanistan, India, Iran, Iraq, Pakistan, Qatar, and Saudi Arabia. Rhazya stricta and its metabolites are used to treat diseases such as cancer, skin diseases, hypertension, rheumatism, sore throat, syphilis, and fever (17). Various studies have shown that different parts of R. stricta contain many phytochemical elements, such as alkaloids, flavonoids, and terpenes (18, 19).
It has been reported that plant species are the same species of the same plant, but in different regions, and even different extracts of the same species have different antibacterial properties (6). Due to the fact that different solvents have different abilities to extract specific types and amounts of phytochemicals of plants (20), in this research different types of solvents were tested. In addition to investigating the abilities of solvents, in extracting phytochemicals, the antimicrobial ability of medicinal plants should also be investigated. It should also be clarified which medicinal plant, with the help of which solvent, can be effective on which microbe.
2. Objectives
We aimed to evaluate the antimicrobial activity of R. stricta extract prepared with different solvents against S. typhimurium.
3. Methods
3.1. Plant Ingredients
Rhazya stricta was collected from Saravan (Iran, Sistan and Baluchistan). The plant species was identified in the botanical laboratory of University of Zabol in 2021.
3.2. Preparation of Extract
Rhazya stricta leaves were dried and ground. Then, 20 g of powdered plant leaves were soaked separately in water, ethanol, methanol, hydroalcoholic (70 alcohols plus 30 water), and ethyl acetate and kept on a shaker for 24 h. After one day, the material was passed through No. 2 filter paper. The solvents were removed from the filtered material by a vacuum rotary apparatus. The concentrated extract was stored in an oven at 40°C for 48 h until a pure extract was obtained and the solvent was removed. Finally, the obtained extracts were dried, and after weighing, they were kept in the refrigerator at 4°C until the experiment.
3.3. Isolation of Salmonella typhimurium from Poultry Feces
Fresh samples of poultry feces were collected from Zabol city (Research Institute of Specific Livestock, University of Zabol). Due to the traditional method for raising poultry in this area and the lack of medication and antibiotics for non-therapeutic purposes, pathogenic bacteria are expected to be highly sensitive to antibiotic treatment. Stool samples were stored at room temperature for 6 h immediately after collection. The samples were transferred to a selective medium for S. typhimurium agar (Salmonella Shigella [SS] agar) and Simon sulfite agar for selective bacterial growth and maintained at 37°C for 24 h. In the next step, using biochemical subjects, culture on mechanical agar, and incubation at 37°C and 42°C, citrate test, motion test, and culture on medium (fermentation and oxidation) containing glucose, the definitive diagnosis of bacteria was made. Then, by culturing bacterial samples grown in the previous stage in the differential culture media of TSI, MRVP, lysine iron agar, Simon citrate, and urea, and with the help of biochemical tests, eight strains of S. typhimurium were isolated and identified (21). All the strains were the same, and no difference was found between them. After the isolation of each strain, the pure strain was obtained in several successive culture stages.
3.4. Inhibitory Growth Diameter Test
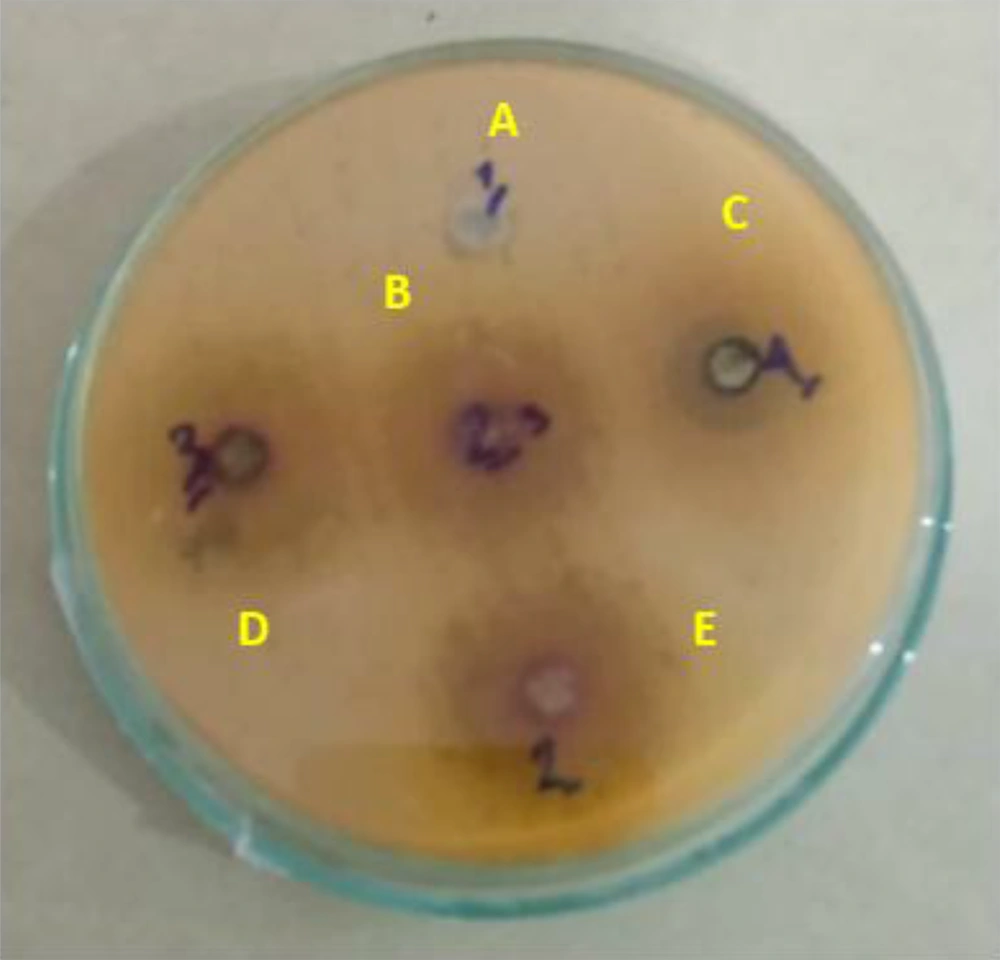
Antibacterial analysis of the diameter of the growth diffusion halo or disk diffusion, which is a method for measuring the antibacterial effects of formulations, is one of the most important and widely used tests in the field of microbiology. In this method, the bacteria were cultured on a plate, and then the test disks were transferred to a plate, and after 24 hours, the area where the bacteria did not grow was calculated (16).
3.5. MIC and MBC of Bactericidal Concentration
The dilution method was used to determine the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of R. stricta extracts. For this purpose, 100 μL of Mueller Hinton broth (MHB) medium was first added to each micro-plate well. Then, 100 μL of diluted solution of each extracts (water, ethanol, methanol, hydroalcoholic, and ethyl acetate) was added to the first well. After mixing, 100 μL was taken from the first well and added to the second well, and this was repeated until the last well. The 100-μL culture medium mixed with the extract was removed from the last well. Then, 10 μL of the microbial suspension containing 108 units/mL (equivalent to 0.5 McFarland) of each S. typhimurium was added separately and incubated at 37°C for 24 h. The first well to inhibit bacterial growth after incubation was considered as the MIC. To ensure clear wells, 10 μL was taken and transferred to Müller Hinton agar medium, and after 24 h, the first dilution that could kill 99.9% of bacteria was determined as the MBC (22).
3.6. Data Analysis
The experiment was performed in triplicate. Statistix version 10 was used for statistical analysis. Mean comparisons were conducted using LSD at 1% probability level. Excel was used to draw the figures.
4. Results
4.1. MIC and MBC of Plant Extracts
The lowest and the highest MICs of the ethyl acetate extract were 25 and 100 ppm, respectively. The lowest and the highest MICs of the hydroalcoholic extract were 3.1 and 25 ppm, respectively (Table 1). The lowest and the highest MICs of the aqueous extract were 6.25 and 50 ppm, respectively. The lowest and the highest MICs of the ethanolic extract were 1.3 and 25 ppm, respectively. The lowest and the highest MICs of the methanolic extract were 12.5 and 50 ppm, respectively. The results showed that the highest and lowest MBCs of the ethyl acetate extract were equal to 200 and 50 ppm, respectively, and the highest and lowest MBCs of the aqueous extract were equal to 100 and 12.5 ppm, respectively. The highest and lowest MBCs of the ethanolic extract were 50 and 6.25 ppm, respectively. Finally, the highest and lowest MBCs of the methanolic extract were 100 and 12.5 ppm, respectively, and those of the hydroalcoholic extract were 50 and 6.2 ppm, respectively (Table 2).
| Bacteria Strains | Hydro-alcoholic | Methanolic | Ethanolic | Aqueous | Ethyl Acetate |
|---|---|---|---|---|---|
| S. typhimurium 1 | 6.25 | 12.5 | 3.1 | 12.5 | 100 |
| S. typhimurium 2 | 12.5 | 25 | 3.1 | 6.25 | 50 |
| S. typhimurium 3 | 3.1 | 25 | 6.25 | 12.5 | 100 |
| S. typhimurium 4 | 25 | 25 | 25 | 50 | 50 |
| S. typhimurium 5 | 3.1 | 12.5 | 6.25 | 25 | 50 |
| S. typhimurium 6 | 6.25 | 50 | 12.5 | 25 | 100 |
| S. typhimurium 7 | 3.1 | 25 | 3.1 | 6.25 | 25 |
| S. typhimurium 8 | 6.25 | 25 | 25 | 25 | 100 |
| Bacteria Strains | Hydro-alcoholic | Methanolic | Ethanolic | Aqueous | Ethyl Acetate |
|---|---|---|---|---|---|
| S. typhimurium 1 | 12.5 | 25 | 6.2 | 25 | 200 |
| S. typhimurium 2 | 25 | 50 | 6.2 | 12.5 | 100 |
| S. typhimurium 3 | 6.2 | 50 | 12.5 | 25 | 200 |
| S. typhimurium 4 | 50 | 50 | 50 | 100 | 100 |
| S. typhimurium 5 | 6.2 | 25 | 12.5 | 50 | 100 |
| S. typhimurium 6 | 12.5 | 100 | 25 | 50 | 200 |
| S. typhimurium 7 | 6.2 | 12.5 | 6.2 | 12.5 | 50 |
| S. typhimurium 8 | 12.5 | 50 | 50 | 50 | 200 |
4.2. Diameter of Inhibition Zone of Plant Extracts Against Salmonella typhimurium
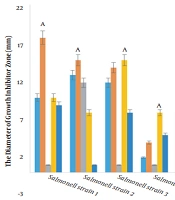
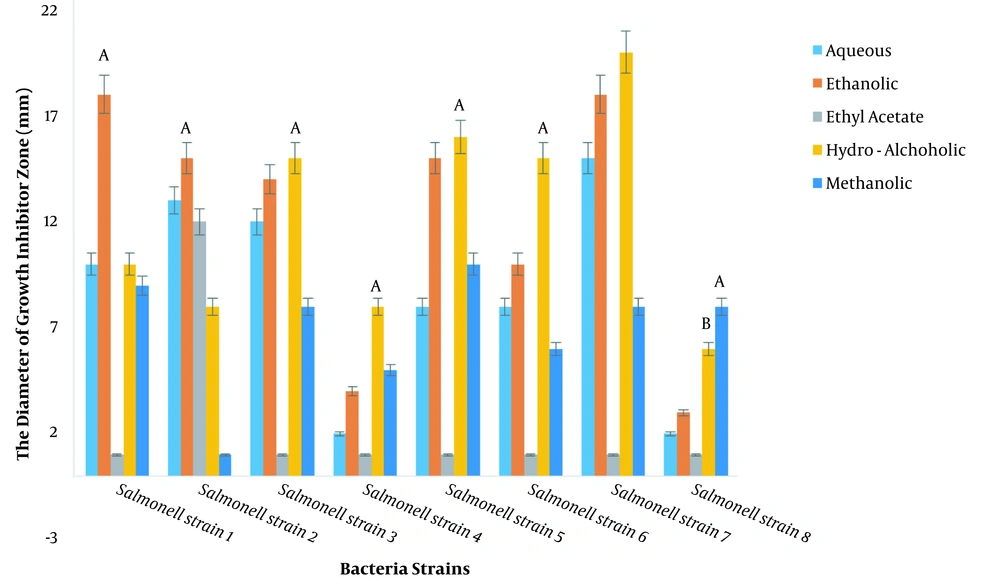
The diameters of the growth inhibition zone of different extracts were investigated at a dilution of 100 ppm against S. typhimurium, and it was found that different extracts had varied effects on the growth inhibition of S. typhimurium (P < 0.01; Figure 1, Table 3). The LSD test showed that among the plant extracts, the most effective extract in inhibiting the growth of S. typhimurium was the hydroalcoholic extract with an average diameter of 12.25 mm of growth inhibition zone, followed by the ethanolic extract with a diameter of no growth of 12.12 mm. Note that the ethyl acetate extract had the least effect (Figure 2).
a Significant at the level of 1%.
5. Discussion
The antibacterial effects of the methanolic and aqueous extracts of Archangel vaccinium was evaluated on S. typhimurium. The results showed that all the strains were sensitive to the methanolic and aqueous extracts of Archangel vaccinia. The average growth inhibition zone diameter ranged from 6.6 to 26.6 mm. The MIC values were between 50 to 200 ppm and MBC values were between 100 and 400 ppm (23). In this study, the lowest MIC of 3.1 ppm was obtained, which had a stronger effect than the Arctostaphylos vaccine.
The antimicrobial properties of the hydroalcoholic extracts of 29 medicinal plants were investigated against E. coli and Staphylococcus microbes. It was concluded that there was a significant difference between the antimicrobial abilities of the hydroalcoholic extracts of medicinal plants in the two methods (6-mm paper discs and the well method), such that the diameter of the growth inhibition zone of both types of bacteria in the well method was larger than that of the paper disk method (24). This study was performed using the disc method, so it is suggested to use the well method in future research to obtain more accurate results.
The antimicrobial properties of the hydroalcoholic extract of Bilher were investigated, and it was concluded that the most acceptable MIC and minimum concentrations of microorganisms were 20 and 40 ppm, respectively (25). In this study, the lowest MIC of 3.1 ppm was obtained, which had a stronger effect than the Dorema aucheri.
The antimicrobial properties of dill and coriander seeds essential oils were investigated using the dilution susceptibility test in liquid medium against S. aureus, E. coli O157: H7, and S. typhimurium. The results showed that S. aureus was the most sensitive and S. typhimurium was the most resistant bacteria to both essential oils. Coriander seed essential oil had a greater inhibitory effect against Gram-negative bacteria than dill seed essential oil. Coriander seed essential oil had MIC and MBC equal to 1000 ppm, and dill seed essential oil had the lowest MIC equal to 500 ppm. The lowest MBC was 1000 ppm against S. aureus. In the case of Salmonella, the essential oils showed no inhibitory effects at any of the concentrations (9). Alum (Ming Fan) was found to have the greatest activity with the mean MIC value of 0.29% (w/v) followed by R. stricta, Juglans regia, and propolis with the mean MIC values of 0.4, 2.66, and 3.75, respectively (26). In this study, the micro-dilution and paper disk methods proved that R. stricta has strong antibacterial properties against S. typhimurium.
The antimicrobial properties of hydroalcoholic extracts of some medicinal plants were investigated against a range of Gram-positive and Gram-negative bacteria. The results have shown that the Myrtle extract with an average growth inhibitor zone diameter of 2.50 ± 0.43, 1.67 ± 0.25, and 2.33 ± 0.66, respectively, was the most effective medicinal plant against of Listeriamonocytogenes, S. typhimurium, and S. aureus. Rosemary extract with an average growth inhibitor zone diameter of 1.25 ± 0.66 cm was the most effective extract against E. coli (10). Rhazya stricta leaves extract was found to be more active as compared to the extract of other parts exhibiting 69.23% and 66.66% activity against Gram-positive and Gram-negative organisms, respectively (27). In this study, it was found that among the plant extracts, the most effective extract for inhibiting the growth of S. typhimurium was the hydroalcoholic extract with an average growth inhibition zone diameter of 12.25 mm. Meanwhile, the ethanolic extract with a growth inhibition zone diameter of 12.12 mm was in the next rank. Thus, it can be stated that the medicinal plant R. stricta has less properties than Myrtus communis.
The anti-cancer properties of A. urmiana have been investigated using S. typhimurium TA100, and the results have shown that A. urmiana in Lake Urmia and other saline waters has a strong anti-mutagenic effect. This anti-mutagenic and anti-cancer effect was indicated 85% against de-capsulated eggs, 70% against incomplete dry artemia, and 100% against cysts. According to Ong's formula, if the anti-mutagenic effect is above 40%, the test substance has a high anti-mutagenic power (11).
The anti-mutation properties of the ethanolic and aqueous extracts of mangrove were investigated against Salmonella mutagenic bacterium TA100. The results revealed that the number of mutated colonies was reduced in the presence of both aqueous and ethanolic extracts with and without metabolic activators. The ethanolic extract showed higher anti-mutagenic activity compared to aqueous extract. No differences in the presence of the S9 mixture were observed between the assays. The highest (71%) and lowest (24%) levels of inhibition of the S. typhimurium mutant were observed in the ethanolic extract of mature leaves of Bardkhoon region and the aqueous extract of young leaves of Assaluyeh (12). In this study, it was found that non-ethanolic extract was had a higher antibacterial effect than the aqueous extract, but in general, the hydroalcoholic extract was the most significant plant extract of Rhazya stricta against S. typhimurium.
The antimicrobial effects of essential oils of four medicinal plants were evaluated against S. typhimurium and compared with the common antibiotics in veterinary medicine. The results demonstrated that Among the essential oils of Thyme essential oil, the combination of thyme essential oil with fennel and the combination of thyme essential oil with marjoram, compared to other essential oils, had a larger diameter of growth inhibition zone. The lowest of MIC for thyme was 156.50 ppm, and its lowest of MBC was 312.50 ppm (13). In addition, the lowest MIC of R. stricta extract against Salmonella was equal to 3.1 ppm.
The antimicrobial effect of sumac essential oil against S. typhimurium was investigated. The results showed that among the components obtained in sumac essential oil, caryophyllene was the most important component with antimicrobial properties, which with its high content in the essential oil composition, can justify its antimicrobial properties. Sumac essential oil has bacteriostatic effects even at low concentrations (30 ppm). With increasing concentration, this effect increased. Therefore, it can be concluded that sumac essential oil can be used to control the growth of S. typhimurium due to its high antimicrobial components (28). However, the lowest inhibitory concentration of R. stricta extract against Salmonella was equal to 3.1 ppm. The antioxidant properties and antimicrobial effects of honeysuckle root extract on foodborne pathogenic bacteria were evaluated, and S. typhimurium and E. coli exhibited greater resistance to the extract (29). The lowest MIC of R. stricta extract against Salmonella was equal to 3.1 ppm.
The higher genotoxicity of T. vulgaris extract was shown by improved tail moments in Comet assay compared to the R. stricta extract and the combined extract (30). The antioxidant and antimicrobial effects of honeysuckle root extract against foodborne pathogenic bacteria and concluded that S. typhimurium and E. coli have shown more resistance to the extract (29), whereas in this study S. typhimurium only in ethyl acetate extract. However, has been shown to be sensitive to other extracts.
5.1. Limitation of Study
For this type of bacteria, the standard type was not available in our laboratory; thus, we had to perform the test without the standard sample.
5.2. Conclusions
The lowest MIC of R. stricta was 3.1 ppm, which had a stronger effect than the Arctostaphylos vaccine. The most effective extract to inhibit the growth of S. typhimurium was the hydroalcoholic extract with an average growth inhibition zone diameter of 12.25 mm. Rhazya stricta plant, with the help of hydroalcoholic solvent can be considered an effective plant for eliminating some bacteria, including S. typhimurium.