1. Background
Having the ability to differentiate into different cellular types and their self-renewal capacities, mesenchymal stem cells (MSCs) are now considered proper tools in cell therapy, as well as the establishment tolerance against transplanted organs. Umbilical cord stem cells have multipotent characteristics and high adhesion properties. Due to the low immunogenicity, the most basic application of Wharton’s jelly-derived MSCs (WJ-MSCs) would be in allogeneic transplantation. Low-level expression of major histocompatibility complex (MHC) class I - but not class II (HLA-DR) - and co-stimulatory antigens (such as CD80 and CD86) are among the reasons for the low immunogenicity of Wharton’s jelly (1-4).
Recent studies have begun to examine MSCs in inflammatory conditions, which could be important in MSC-mediated cell therapies. MSCs are widely used in the treatment of immunological and non-immunological diseases due to their modulating properties through factors that suppress the immune system and their self-renewal capacities (5).
Interferon gamma (IFN-γ) is a pro-inflammatory cytokine largely spattered during infection or inflammatory responses (6-10). Although low levels of IFN-γ stimulate the presentation of antigen in MSCs and result in pro-inflammatory responses, high levels of IFN-γ indirectly reduce MHC class II expression (11, 12), suggesting its potential to be used as a supplement to reduce the immunogenicity of transplanted cells. In this regard, previous studies have shown that IFN-γ leads to suppression of T cell proliferation in the presence of MSCs (13, 14).
At molecular levels, nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), a family of cytoplasmic proteins, can identify pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), which set up inflammation (15). In order to inhibit and/or modulate the inflammatory response, NLR expression can be adjusted during antigen presentation from microorganisms or in response to cellular damages.
Following the identification of PAMPs, pattern recognition receptors (PRRs) are induced and lead to the production of cytokines and other mediators of innate immunity via activation of nuclear factor-κB (NF-κB) and activating protein 1 (AP-1) signaling pathways.
Some NLRs (such as NOD1, NOD2, and NLR family pyrin domain containing 3 [NLRP3]/cryopyrin) cause the secretion of pro-inflammatory cytokines, while some others (such as PYPAF2, PYPAF, LDL receptor related protein 10 [LRP10]/PYNOD, NLR family CARD domain containing 5 [NLRC5], and NLRP12/Monarch-1/PYPAF7) inhibit apoptosis-associated speck-like protein containing a CARD (ASC)-mediated NF-κB activation and the secretion of caspase-1-dependent pro-inflammatory cytokine (16).
Previous studies have shown that NLRC3 plays an inhibitory role during infection and inflammation by inhibiting the activity of NF-κB, as NF-κB activity is limited following stimulation with lipopolysaccharides (LPS) (17).
Moreover, it has been reported that NLRC5 expression can be induced in many immune cells by various stimulators. IFN-γ is capable of strongly inducing NLRC5 expression at both messenger RNA (mRNA) and protein levels in different cell types, including lymphocytes (CD4+ and CD8+ T cells), myeloid cells (macrophages or dendritic cells), fibroblasts, and Hela cells (15, 18). NLRC5 is also able to inhibit the NF-κB pathway by blocking phosphorylation of 2 subunits of IκB kinase (IKK), IKKA and IKKB. Several studies have demonstrated that NOD1 and NOD2 lead to upregulation of NF-κB signaling (17, 18). It is proven that activation of MSCs using toll-like receptor 3 (TLR3) and TLR4 agonists leads to the creation of anti-inflammatory (MSC2) and pro-inflammatory (MSC1) phenotypes, respectively (19).
2. Objectives
In this study, according to the immunosuppressive properties of WJ-MSCs and characterization of NLRC3 and NLRC5 genes, we aimed to investigate the expression of these genes in both untreated and IFN-γ–treated WJ-MSCs so that the IFN-γ treatment may reduce the immunogenic properties of transplanted cells through upregulation of these genes. Finally, the results may suggest that MSC cellular therapy could reduce transplant rejections and promote tissue repair via the modulation of immune responses mediated by IFN-γ treatment. However, much more effort is needed to fully evaluate the practical aspects of this application.
3. Methods
All samples were collected from subjects using standard methods and following informed consent. The project was approved by the Ethics Committee of Mohaghegh Ardabili University (UMA.REC.324724).
3.1. Isolation and Culture of Mesenchymal Stem Cells from Human Umbilical Cord Wharton’s Jelly
Following written consent signed by their parents, umbilical cord blood samples were obtained from healthy newborns immediately after cesarean sections (Sabalan Hospital) and transferred to the laboratory in normal saline. Isolation of human umbilical cord cells was performed after a standard tissue culture. For this purpose, the cords were washed with 70% alcohol and cut into 2 cm pieces in Hanks’ balanced salt solution (HBSS) after removal of the vessels. Wharton’s jelly was chopped into pieces of approximately 0.5 mm by a scalpel (19). Tissue pieces were then cultured in low-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Germany), supplemented with 20% fetal bovine serum (FBS; Gibco, Germany), 1% penicillin/streptomycin (Sigma, USA), and 1% amphotericin B (SIGMA, America), and incubated at 37°C with 5% CO2.
After 3 days, the cultures were observed regarding possible contamination or media color changing (19). The cultures were then placed again in an incubator for 10 days without manipulations. After observing the first MSCs, the culture medium was then replaced every 3 days. When the culture reached 70 - 80% confluency, the cells were harvested with a 0.25% trypsin-EDTA (Gibco, England) and passaged to new flasks (20).
3.2. Flow Cytometry Analysis in Wharton’s Jelly Stem Cells
The immunophenotyping of isolated MSCs was assessed by flow cytometric analysis to confirm cell identities. In order to stain for surface markers CD73 and CD105, the cells were dissolved in the stain solution. A cell suspension containing 1 × 106 cells per mL was provided. The cell suspension was then added to a new Eppendorf tube for flow cytometry. The specific antibodies were then added to each tube according to the manufacturer’s recommendation. The cells were incubated with antibodies in the stain solution for 45 minutes at 4°C in a dark place. The same process was performed for isotype controls. Finally, the cells were analyzed by a BD FACSCalibur flow cytometer (BD Biosciences, USA), and the results were analyzed using FlowJo 7.6.1 (FlowJo LLC, USA) (21).
3.3. RNA Isolation and Complementary DNA Synthesis
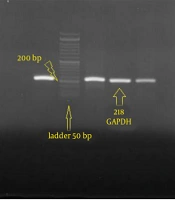
Total RNA was isolated from both untreated and IFN-γ–treated cells (24 hours after treatment) using the ONE STEP-RNA Reagent Kit (BIO BASIC, Canada) according to the manufacturer’s protocol. In order to remove genomic contamination, RNA was treated with deoxyribonuclease (DNase) I (Thermo Fisher Scientific, USA) according to the protocol described by the manufacture. The quantity of extracted RNA was evaluated by calculating the 260/280-nm wavelength ratio. The quality was confirmed by electrophoresis in agarose gel. The extracted RNA samples were then diluted to 400 ng/µL concentration for the complementary DNA (cDNA) synthesis procedure. Reverse transcription was performed using the PrimeScript RT Reagent Kit (Takara, Japan) according to the kit instructions (Figure 1) (22).
3.4. Quantitative Real-time Polymerase Chain Reaction
The mRNA quantitative expression of NLRC3 and NLRC5 genes was measured in the samples treated with or without IFN-γ using the SYBR Premix Ex Taq II Kit (Takara, Japan) according to the manufacturer’s instructions. Briefly, each polymerase chain reaction (PCR) contained 400 ng/µL of cDNA, 10 µL of SYBR Mastermix, 0.5 µL of forward primer, 0.5 µL of reverse primer, and deionized water in a total volume of 20 µL. The PCR cycles were performed at 95°C for 10 minutes, followed by 45 cycles of 95°C for 10 seconds, an annealing temperature for 30 seconds, and at 72°C for 20 seconds. A melting temperature profile was conducted at 55 to 95°C to form a melting curve chart. Real-time PCR for each gene was performed in duplicate using a Rotor-Gene 6000 Thermocycler (Qiagen, Netherlands). The obtained cycle threshold (CT) values were used to analyze expression levels using the 2-ΔΔCt method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference gene to normalize gene expressions. The oligonucleotide sequences of the synthesized primers are given in Table 1 (23, 24).
| Gene | Primer Sequence (5’ – 3’) | Size | Annealing Temperature (°C) |
|---|---|---|---|
| NLRC3 | 160 bp | 60 | |
| F | ATTCTCTGGGGATGGACG | ||
| R | ATTTCAACAGTGCACGTG G | ||
| NLRC5 | 173 bp | 59 | |
| F | TGGAGAAGAATCAGATCACAG | ||
| R | GGG CTG GTT GTC AAA GAA | ||
| GAPDH | 218 bp | 59 | |
| F | GAAGGTGAAGGTCGGAGTC | ||
| R | GAAGATGGTGATGGGATTTC |
Primer Sequences Used in Real-time Polymerase Chain Reaction Assays
3.5. Statistical Analysis
Data analysis was performed using GraphPad Prism 5.04 (GraphPad Software, USA). The data were analyzed using Kruskal-Wallis and Mann-Whitney tests. The data were indicated as mean (SD). All the performed tests had the confidence of 95%. A P value less than 0.05 was considered statistically significant.
4. Results
4.1. Evaluation of Wharton’s Jelly-Derived Mesenchymal Stem Cell Morphology and Immunophenotype
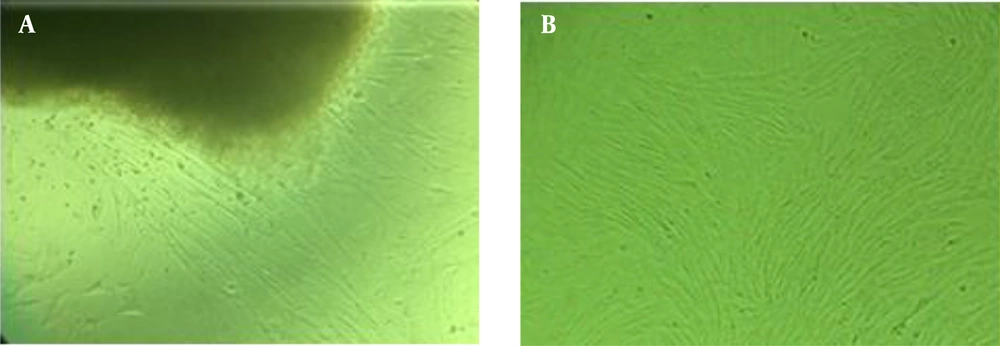
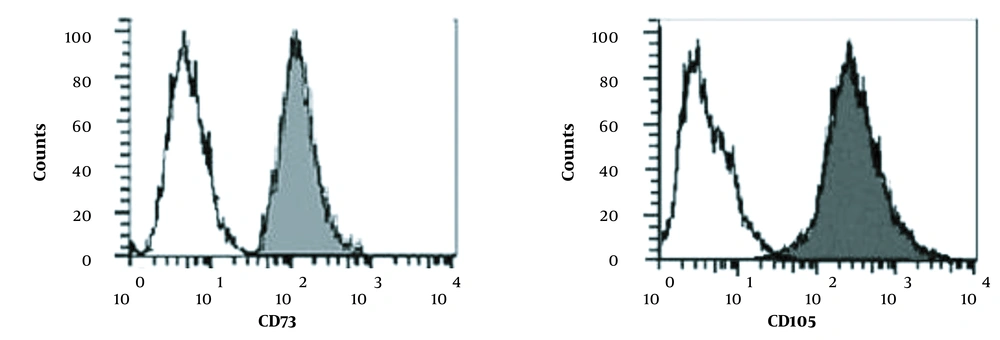
Ten to fourteen days after putting the pieces of Wharton’s jelly in culture, cell buds began to grow out of the sides and a week later covered the flask floor. During all phases, cord-stromal cells were examined using an inverted microscope (Olympus, Japan). The cells resemble fibroblasts with frills drawn around the flask floor (Figure 1). When 80% of the flask floor was occupied by attached fibroblasts, cell passaging was performed into several flasks. The grown MSCs from the first passage of the human umbilical cord are shown in Figure 1. The flow cytometry results are also indicated for both CD73 and CD105 surface expression in isolated WJ-MSCs, demonstrating a significant upregulation of the suspected markers in isolated cells (Figure 2). Although in the majority of studies, surface markers CD34, CD45, CD73, CD90, and CD105 are utilized to identify MSCs, in the current study, due to some limitations, we only used CD73 and CD105.
Morphological characteristics of mesenchymal stem cells isolated from Wharton’s jelly. A, primary culture after 10 - 14 days; cell buds’ growth from the sides of Wharton’s jelly tissue (magnification × 40); B, after the first passage, the cells, which resemble fibroblast cells with frills drawn around them, were stuck to the flask floor (magnification × 40).
4.2. Qualitative Evaluation of RNA and Reverse Transcriptase-Polymerase Chain Reaction Reviews
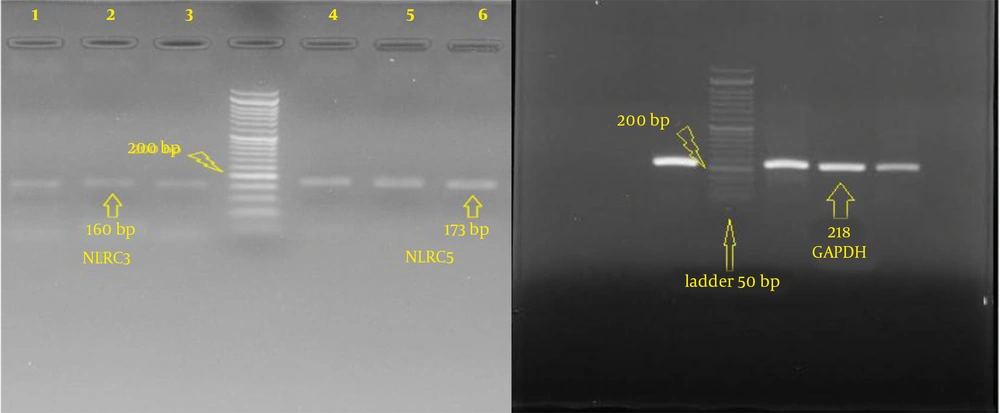
The qualitative results of RNA extracted from Wharton’s jelly showed typical bands representing 18S rRNA and 28S rRNA (Figure 3). All cDNAs synthesized with NLRC3, NLRC5, and GAPDH primers were amplified using reverse transcriptase PCR (RT-PCR). Also, cDNA synthesized with NLRC3, NLRC5 and GAPDH designed primers was tested for Wharton’s jelly stem cells, and the bands were observed in 160 base pairs (bp), 173 bp, and 218 bp, respectively (Figure 4).
4.3. Real-time Polymerase Chain Reaction Results
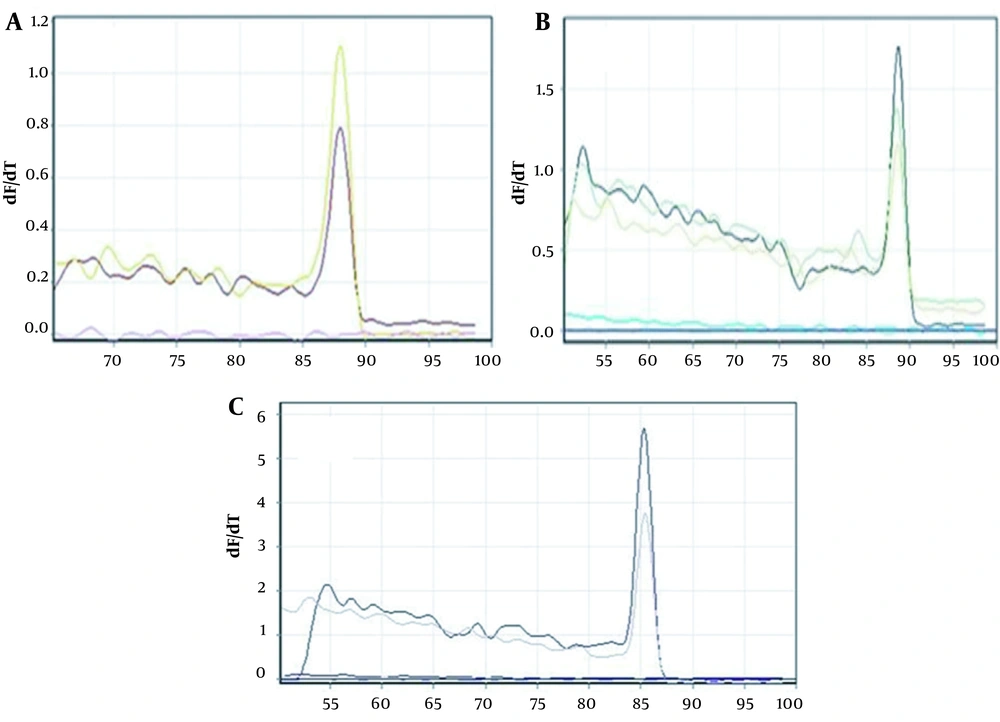
The expression of NLRC5 and NLRC3 genes in both untreated (control) and IFN-γ–treated WJ-MSCs was measured 24 hours after treatment using real-time PCR relative quantitation. The expression of the target gene was calculated using 2-ΔΔCt and GAPDH as an internal control (Figure 5).
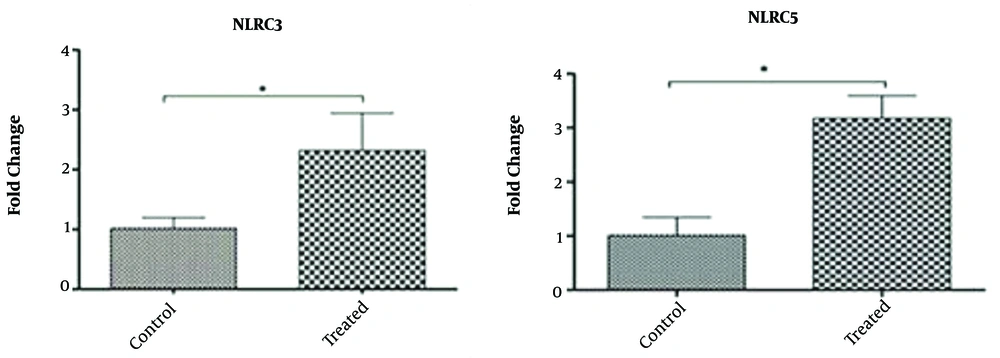
As shown in Figure 6, there is a significant difference in the expression of NLRC3 and NLRC5 genes in treated and untreated WJ-MSCs (P ≤ 0.05). The expression of these target genes in treated samples showed a significant increase compared to untreated WJ-MSCs, implying the modulation of gene expression through treatment with IFN-γ cytokine. The melting curve for each product represents a single PCR product peak. The standard curves had a slope of -3.2, indicating efficient PCR.
5. Discussion
Although the immunosuppressive role of stem cells has been confirmed in various studies, more implications of these cells for the treatment of diseases require greater recognition of precise mechanisms beyond their functions. In this study, NLRC3 and NLRC5 gene expressions were examined in IFN-γ-treated WJ-MSCs. Due to their capacity for renewal and differentiation into other cell lines, WJ-MSCs have broad therapeutic potential for regenerative medicine and cell-based therapies. However, some problems (such as stimulating the recipient’s immune system) have always been a major stumbling block in this regard. As in 1985, the bone marrow stromal cell system was first described by Owen and Friedenstein in connective tissue engineering, cell transplantation, hematopoietic stem cell transplantation, and gene therapy. Their research results extensively demonstrated the osteogenesis potential in cultured cells from bone marrow (25).
NLRs are a batch of PRRs expressed in non-immune cells (including epithelial, mesothelial, and immune cells), which play key roles in immunity and regulate antigen presentation (NLRC5 and class II major histocompatibility complex transactivator [CIITA]) of pathogens and/or damaged cells (NLRP1, NLRP3, and NRC4) to suppress or modulate inflammation (NLRC3, NLRP6, NLRP12, and NLR family member X1 [NLRX1]) (26). Among the NLRC subfamily, studies have shown that NLRC1 (NOD1), NLRC2 (NOD2), and NLRC4 play important roles in the host defense against bacterial pathogens (27, 28). NLRs are expressed in non-immune cells, including epithelial, mesothelial, and immune cells. Studies have shown that NLRs are also involved in multiple non-inflammatory roles, as NLRC1 and NLRC2 regulate the differentiation of human umbilical cord blood-derived MSCs (29).
In this study, isolation of Wharton’s jelly stem cells from human umbilical cords of healthy newborns was performed using the tissue culture method, as described in previous studies (30, 31). The Wharton’s jelly stem cells were observed on days 10 - 14. The relatively low number of cells was seen to form long and flattened colonies. This was in accordance with the findings of Gronthos and Simmons and Kuznetsov et al. (32, 33). Then, these cells were rapidly proliferated over time, and the number of cells increased, which forced us to passage into the flasks (19, 33).
Recent studies have identified NLRC5 as an immune regulator. NLRC5 regulates MHC class I gene expression and could be a potential target for transplant rejection and cancer immunotherapy (34). NLRC5 has high levels of expression in immune cells, including macrophages derived from bone, splenic dendritic cells, CD4+ T cells, CD8+ T cells, and B cells (15). Harton et al. showed CLR16.2 (a partial sequence from NLRC3) expression in a variety of human immune cell classes, especially in T cells, while its expression seems insignificant in epithelial cell lines (35, 36). Conti et al. suggested that CLR16.2 plays a role in the reduction of T cell activation (37), proposing its potential to efficiently modulate immune responses. Also, Schneider et al. displayed that NLRC3 inhibits TLR4-mediated NF-κB activation and inflammation (17). Biswas et al. and Yao et al. found NLRC5 as an important regulator of MHC class I genes, promoting the host defense (38, 39).
Our results showed that NLRC3 and NLRC5 genes were expressed at low levels in untreated WJ-MSCs cells (control), while IFN-γ–treated WJ-MSCs were highly enriched in their mRNA transcripts. The results are consistent with previous data, showing that NLRC5 mRNA and protein were strongly induced in the mouse macrophage cell line when the cells had been treated with lipopolysaccharide. Thus, it is concluded that NLRC5 might be a negative regulator of NF-κB and type I interferon signaling pathways, playing an important role in modulating innate immunity (40). Meissner et al. reported that NLRC5 was rapidly induced when cells were exposed to IFN-γ, while induction of MHC class I molecules was occurred later (41). In line with our study, Kuenzel et al. reported that the expressions of both NLRC5 and CIITA were highly induced after IFN-γ stimulation (42). Recently, it has been demonstrated the regulatory role of NLRC5 in IFN-c through MHC class I-mediated CD8+ T cell activation, followed by the IFN-c–mediated upregulation of NLRC5 expression as a positive feedback loop for the promotion of MHC class I-dependent immune responses (43).
NLRC3 expression has been shown in T lymphocytes and other immune cells. Its inhibitory role in T cell activation has been reported in response to stimulation with anti-CD28 and anti-CD3 antibodies (37). NLRC3 interaction with target proteins leads to the weakening of the immune response in several important inflammatory pathways (16). It is suggested that competition between NLRC3 and inflammatory components appears as a brake on the synthesis of pro-inflammatory cytokines and probably their secretion (44). Zhang et al. demonstrated that NLRC3-knockout mice infected with herpes simplex virus (HSV) showed an increase in the innate immune response as compared to wild-type mice (44). In addition, Gultekin et al. proposed that NLRC3 overexpression could negatively regulate inflammatory responses via the NF-κB signaling pathway and/or inflammasome complex formation (16).
In another study, Shiau et al. found that NLRC3 mutation led to the activation of primitive macrophages associated with systemic inflammation, increased pro-inflammatory cytokines, and macrophage aggregation rather than their migration into the brain to form microglia (45). Dysregulated innate immunity has been indicated to result in many inflammation-associated diseases. Thus, the growing recognition of the molecular mechanisms regulating negatively innate immunity appears to be useful in developing novel and more effective treatments to prevent inflammation-induced autoimmune diseases and cancer. Altogether, we would be interested in investigating other family members of NLRCs genes, which may be involved in the immunomodulatory properties of MSCs.
5.1. Conclusion
The effect of treatment with IFN-γ was led to increased expression of NLRC3 and NLRC5 genes in IFN-γ–treated WJ-MSCs. Therefore, it may be suggested as a proper source for cell therapy, especially in inflammatory conditions.