1. Background
Multiple sclerosis (MS), which is a T-cell immune-mediated disease, is mainly characterized by demyelination of the central nervous system (CNS). The typical course of the disease consists of recurrent episodes of neurological disorders that reflect immunological alterations. Variable degrees of relative clinical remission are associated with clinical manifestations of the disease. The neurological signs of the disease are bouts of the inflammatory reaction of the CNS and they reflect the degenerative aggression against a variety of functional neurological systems over the long term (1, 2). MS may follow several different development patterns and variable levels of disability, which often makes early and precise diagnosis difficult (3). Based on genetics, many genes have been identified that develop MS, which can be divided into 2 categories, HLA and Non-HLA. HLA genes such as HLADR2/DQ6, HLADR3/DQ2, and HLADR4/DQ8 have shown a strong correlation with MS (4). From Non-HLA genes, cytokines category, cytokine receptors, and molecules involved in cell signaling process can be mentioned. Since the immune system has an important role in the incidence of this disease, studying factors such as cytokines (IL) is essential (5, 6). Cytokines are involved in almost every part of immunity and inflammation. Finding out the function of individual cytokines is extremely complicated due to the fact that their role can change depending on the immune response phase, the cellular source, and target. In fact, many cytokines have both pro-inflammatory and anti-inflammatory potentials, with the contrasting results determined by the immune cells and their state of responsiveness to the cytokine. These issues make studying the cytokine biology daunting, particularly for IL-10 and IL-10-related genes. The IL-10 superfamily is extremely pleiotropic; some cytokines of which include: IL-26, IL-24, IL-22, IL-20, and IL-19. These genes are related through genetic similarities and intron-exon gene structures. Significant harmony exists not only in shared receptors but also in conserved signaling pathways. However, its members mediate varied activities, including immune suppression, enhanced antibacterial and antiviral immunity, antitumor activity, as well as elevation of self-tolerance in autoimmune diseases (5). IL-19 is primarily made by monocytes, where its expression can be induced by LPS, IL-4, and GM-CSF. IL-19 signals through a receptor complex composed of the IL-20R1 as well as IL-20R2 chains and activate monocytes in an autocrine and paracrine way to release the cytokines IL-6, TNF-α, and numerous ROSs. Roles for IL-19 have primarily been considered in psoriasis and allergic disorders. Patients with psoriasis have increased levels of IL-19 in basal and suprabasal keratinocytes of the involved skin, where it is thought to contribute to the inflammatory process (5-7). The human IL-20 gene is on chromosome 1q32, which consists of IL-10, IL-19, and IL-24 as well (7). Although IL-10 and IL-20 are parts of the same family, they are highly different in sequence and function. Although various cell types express IL-20, it is mainly expressed by monocytes and skin keratinocytes. IL-20 protein is also found at high levels in the skin of patients with psoriasis. Just as IL-19, IL-20 signals through the IL-20R1/IL-20R2 heterodimer; however, IL-20 also binds to the receptor complex composed of IL-22R1 and IL-20R2. Each of these heterodimer receptor complexes functions partly through the Jak-STAT pathways. Furthermore to a role in psoriasis, IL-20 has been found in higher concentrations in synovial fluid from patients with rheumatoid arthritis (8). Other studies have also proposed a potential role for IL-20 in atherosclerosis and angiogenesis (7, 9). To understand the possible role of IL-19 and IL-20, which are anti-inflammatory cytokines in multiple sclerosis, we analyzed their important polymorphisms in multiple sclerosis and compared them to the healthy group in this leading research.
2. Methods
2.1. Patients and Samples
The study was approved by the ethics research committee in Sistan and Baluchestan University, Faculty of Science, and was conducted with clinical samples from MS patients (N = 100, 67 female and 33 male, age: 16 to 79 years, mean = 32.10 ± 35.31), healthy controls without any specific systemic disease (N = 100, age: 16 to 58years, 57 female and 43 male, mean = 32.8 ± 97.27), and from volunteer blood donors who were selected from August 2013 to February 2014. Patients were rejected if they had a history of inflammatory diseases or received anti-inflammatory medicines. All patients were informed of the study and contributed voluntarily. Written consents were taken. The related data regarding the age and gender of both patient and control groups is represented in Table 1.
| Characteristic | Case | Control | |||
|---|---|---|---|---|---|
| Mean (± SD) | Range | Mean (± SD) | Range | P Value | |
| Age | 35.31 ± 32.10 | 16 - 79 | 97.27± 32.8 | 16 - 58 | 0.01 |
| Gender | M = 33, F = 67 | - | M = 43, F = 57 | - | 0.14 |
2.2. Blood Collection and DNA Extraction
Whole peripheral blood (10 mL) samples were taken from all individuals and collected in tubes containing 0.5 M EDTA separately, centrifuged for 15 min at 150 g at 20°C, and then serum stored at 20°C into tubes for DNA extraction. Genomic DNA was extracted from the serum of 100 ounce of MS disease and 100 ounce of healthy group by the DNA extraction kit (DIAtom DNA Prep.). DNA quality extracts were examined by electrophoresis and DNA concentrations of about 60 ng/µL obtained by NanoDrop and ratio of 260/280 nm around 1.7 to 1.9 was accepted.
2.3. PCR Analysis
The rs2243158 and rs2243168 on IL-19 and rs2981572 and rs2981573 on IL-20 were explored through Tetra- primer ARMS-PCR method. PCR amplifications were done in a final volume of 20 µL comprising, 10 µL master mix (TAKARA), 0.7 µL (10 pmol) of each primer, 2 µL template DNA, and 6.8 µL DNase-free water was used. For rs2243158 and rs2243168 the amplification was performed with an initial denaturation step at 95°C for 5 minutes; followed by 38 cycles at 95°C for 30 seconds, 63°C and 60°C for 30 seconds, and 72°C for 30 seconds, respectively, with a final extension at 72°C for 10 minutes. For SNP rs2981572 and rs2981573 the cycling conditions were as follow: an initial denaturation step at 95°C for 5 minutes, followed by 30 cycles at 95°C for 30 seconds, 55°C and 58°C for 30 seconds, and 72°C for 30 seconds, respectively, with a final extension at 72°C for 10 minutes. The PCR products were checked for size and purity by 1.5% agarose gel electrophoresis. The set of primers used to genotypes, IL-19 and IL-20 are listed in Table 2.
| Variables | Primers of SNPs | Length of PCR Product, bp |
|---|---|---|
| IL19, rs2243158 GC | Forward inner (C allele), 5’-GGTGGATCCACCCAGCAAACCTTCAC-3’ | 253 |
| Reverse inner(G allele), 5’-TTTTATTCAGGTGGATAAGAGGAAATGGTC-3’ | 290 | |
| Forward outer, 5’-GCCACAGCTCTCAGGAAAGTGACCTAAG-3’ | 487 | |
| Reverse outer, 5’-CCAGCATCTGGAACATCATAGCCATACA-3’ | ||
| IL19, rs2243168 AT | Forward inner primer, (T allele), 5’-GGAAGTTGCCAAGCTGCCCTCTATCT-3’ | 215 |
| Reverse inner primer, (A allele), 5’-CAATAAGGAGCTAGGGGAAGAAGCCGAT-3’ | 167 | |
| Forward outer primer, 5’-AGAAGGGTAAGAGAATGAGAAGCGGTGG-3’ | 328 | |
| Reverse outer primer, 5’-TGGTTTTTGATGTTTGCCCCTGAAATAA-3’ | ||
| IL20, rs2981572 | Forward inner primer, (T allele), 5’-TTGTCATAAGCTTTTTAATTCATTCTT-3’ | 156 |
| Reverse inner primer, (G allele), 5’-CAAGATAAAAATATTTTAGTGCAATGTC-3’ | 219 | |
| Forward outer primer, 5’-ACTCATCAATAATATTTTCATCATATGCT-3’ | 320 | |
| Reverse outer primer, 5’-AGTTTTAAGATAAAATAATAATGGGCTG-3’ | ||
| IL20, rs2981573 | Forward inner primer, (A allele), 5’-CCTCTCCTAGCTGAGATGAACTGAA-3’ | 181 |
| Reverse inner primer, (G allele), 5’-CTCTTTCAGACCTCACATTTGGAATAAC-3’ | 255 | |
| Forward outer primer, 5’-TCTGAATAGGACCTAGGAATTCAATTCTTT-3’ | 382 | |
| Reverse outer primer, 5’-ATGCTGAAAAGGACCCAAAGAATAATAG-3’ |
2.4. Statistical Analysis
SPSS version 19.0 (SPSS, Chicago) was used for all the statistical analyses. The association between IL-19 and IL-20 with multiple sclerosis was estimated by using the odds ratio (OR) and 95% confidence intervals (95% CI) from logistic regression analyses. The Hardy-Weinberg Equilibrium (HWE) was tested with the X2 test for any of the SNPs under consideration. The significance level was set at P ≤ 0.05 for all tests.
3. Results
3.1. Tetra ARMS PCR Results
After DNA extraction and making sure of the purity and optimal quality of the extracted DNAs, PCR tetra ARMS was performed on them for the 4 selected SNPs. The data and results related to 4 SNPs were given to the SPSS Ver. 22 and the Chi-square test was used to compare the data related to the case and control groups. Statistically the meaningful level was considered to be p≤0.05. rs2243158 (GC) as well as rs2243168 (AT) on IL19 and rs2981572 (1053TG) as well as rs2981573 (1380AG) on IL20 were selected. The outer primers amplify the control fragment, which is necessary for making sure of reaction accuracy. Internal primers amplify fragments that define the different alleles on polymorphisms.
3.2. Gel Electrophoresis and PCR Results
rs2243158 (GC): the control band with the length of 487 bp. The inner primers amplify the G allele fragment (290 bp) and C allele fragment (253 bp). Therefore, if there are any 2 alleles, both of these fragments are produced and appear to be heterozygous (290 bp, 253 bp and 487 bp). rs2243168 (AT): in order to amplify the SNP, the outer primers produced the control band with 328 bp and the inner primers amplified the 215 bp (T) and 167 bp (A) fragments. In this condition, both A and T bands are observable besides the control band (215 bp, 167 bp, 328 bp), which proves this polymorphism to be heterozygote. rs2981572 (1053TG): the length of the control fragment amplified by the external primers, was 320 bp. The internal primers produced the 156 bp (T) and 219 bp (G) fragments. The heterozygotes demonstrated 156 bp, 219 bp, and 320 bp bands. rs2981573 (1380AG): the control fragment was 382 bp. Other fragments produced by internal primers were 181 bp (A) and 255 bp (G). In heterozygotes, fragments with the length 328 bp, 181 bp, and 255 bp were observed.
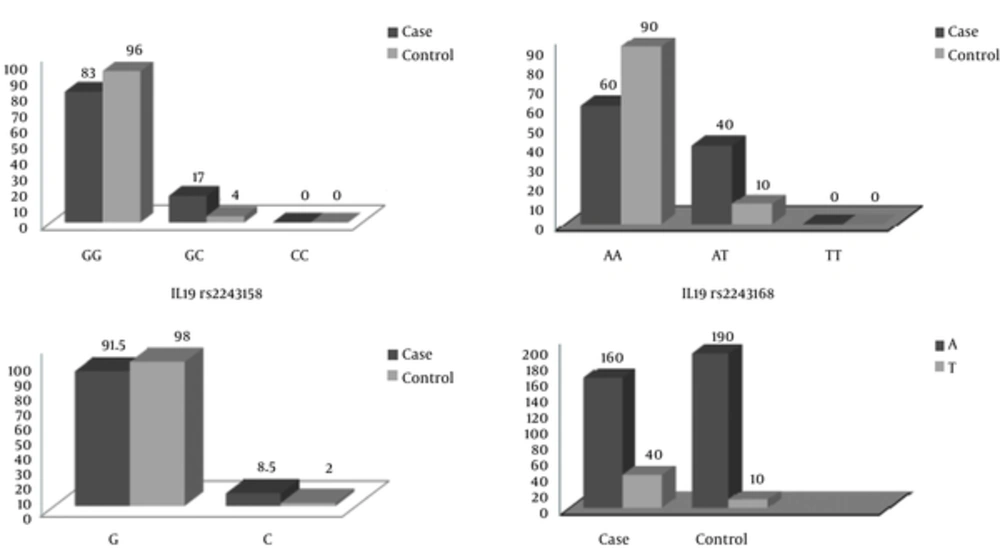
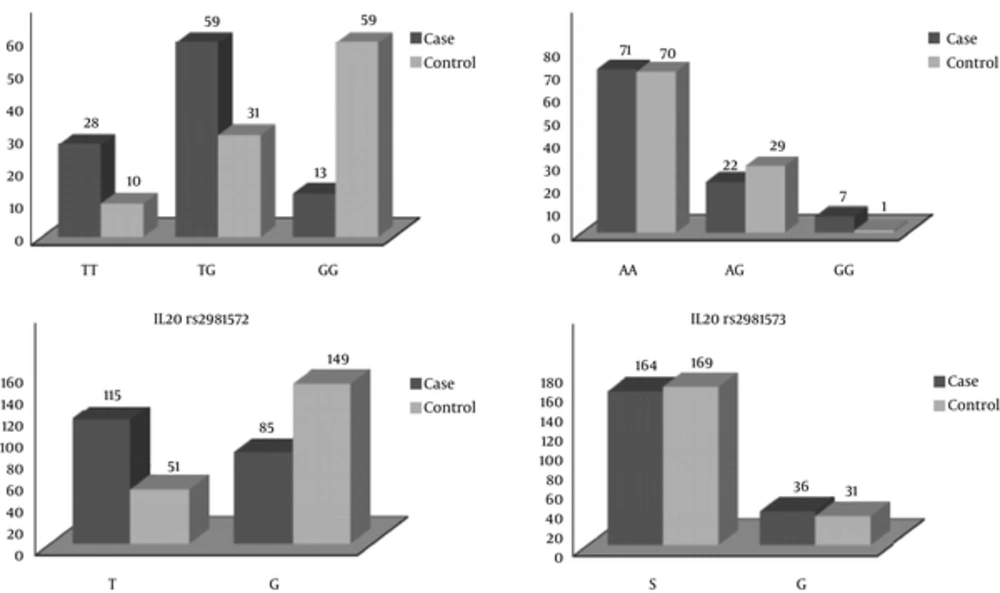
As shown in Table 3, the frequencies of the genotypes and Allelic frequency in both case and control groups and comparison between them.
| Variables | Cases, N = 100 | Control, N = 100 | OR | CI (95%) | P Value |
|---|---|---|---|---|---|
| IL19rs2243158 | |||||
| GG | 83 (83) | 96 (96) | - | - | Ref |
| GC | 17 (17) | 4 (4) | 0.2 | 0.06 - 0.62 | 0.003 |
| CC | 0 | 0 | - | - | - |
| G | 183(91.5) | 196 (98) | - | - | Ref |
| C | 17 (8.5) | 4 (2) | - | - | 0.004 |
| IL19rs2243168 | |||||
| AA | 60 (60) | 90 (90) | - | - | Ref |
| AT | 40 (40) | 10 (10) | 0.16 | 0.07 - 0.35 | 0.001 |
| TT | 0 | 0 | - | - | - |
| A | 160(80) | 190 (95) | - | - | Ref |
| T | 40 (20) | 10 (5) | - | - | 0.001 |
| IL20rs2981572 | |||||
| TT | 28 (28) | 10 (10) | - | - | Ref |
| TG | 59 (59) | 31 (31) | 1.47 | 0.63 - 3.41 | 0.03 |
| GG | 13 (13) | 59 (59) | 12.65 | 4.97 - 32.25 | 0.001 |
| T | 115(57.5) | 51 (25.5) | - | - | Ref |
| G | 85 (42.5) | 149 (74.5) | - | - | 0.001 |
| IL20rs2981573 | |||||
| AA | 71 (71) | 70 (70) | - | - | Ref |
| AG | 22 (22) | 29 (29) | 1.33 | 0.07 - 2.55 | 0.37 |
| GG | 7 (7) | 1 (1) | 0.14 | 0.01 - 1.2 | 0.04 |
| A | 164 (82.5) | 169 (84.5) | - | - | Ref |
| G | 36 (18.5) | 31 (15.5) | - | - | 0.503 |
aValues are expressed as No. (%).
4. Discussion
Multiple sclerosis is an inflammatory disease of the CNS caused through impairment of the body immune system and its activation against its own cells, which is the most prevalent neurologic disease among North European young people. The onset age of the disease is often in the second and third decade of life. Analyzing the epidemiologic, statically, immunological, and genetically studies, one can suggest that MS is a complicated disease in which many factors play roles. From a genetically view point, most of the genes showing relation with MS are among the MHC family.
Among the factors playing roles in inflammation and demyelination are cytokines, which are responsible for regulating and controlling the natural and acquisitive immune responses (10).
This study would be the first one ever performed on the relationship between the polymorphism IL19/IL20 and MS disease; therefore, due to the limited comparative studies, referring to similar studies would be inevitable. IL19 plays a fundamental role in aggravating psoriasis disease, which increases the multiplication of keratinocytes and causes skin problems (dermatological disorders). Increase in IL19 and its receptor chains expression has been detected in psoriasis disease (11). According to the performed analyses, IL19 plays a role in aggravating asthma since mouse lungs subject to allergens. It has been increased in children with asthma compared to healthy children (11). Blumberg et al. analyzed the effect of the increased IL20 expression on the transgenic mouse keratinocytes. The research results confirmed that IL20 expression increase would cause damages similar to the dermatological disorders in the inflammatory disease of psoriasis (12).
The relationship between the polymorphisms IL19 and IL20 with kidney reflex disease has been analyzed by Kurdi et al. and the results signified no meaningful relation between these polymorphisms and the disease (13). Many studies have been performed on the inflammatory disease such as rheumatoid arthritis in which IL20 has a preinflamatory role and aggravate the inflammation (14). IL19 plays a role in rheumatoid arthritis and sceptic shock as well. In another study on the American-European population, a meaningful relation has been observed among some SNPs in IL19 and chronic hepatitis B.
In this research, the state of 2 SNPs of IL19 (rs2243158 GC and rs2243168 AT) and also 2 SNPs of IL20 (rs2981572, 1053TG and rs2981573, 1380AG) as well as their association with MS were studied in both patient and healthy groups. Figures 1 and 2.
The CC genotype was not observed in this study. A meaningful association existed between the GC genotype frequency and MS disease (P = 0.004, OR = 0.2, CI = 0.06 - 0.62), which proved this genotype as a protective factor for the illness. In fact, the individuals carrying GC genotype have 0.2 higher chance of MS disease. G allele, which is a risk factor for MS disease, was more frequent than C allele in both healthy and patient groups (P = 0.004, OR = 4.54, CI = 1.5 - 13.69). rs2243168 (AT): AA genotype was the most frequent genotype in both patient and healthy groups while TT genotype was observed in neither group. There was a meaningful association between AT genotype and the MS disease (P < 0.001, OR = 0.16, CI = 0.07 - 0.35), which candidates this genotype as a protective factor against the disease. The A allele was more frequent than T allele in both groups (P < 0.001, OR = 4.73, CI = 2.30 - 9.80). This allele has a meaningful association with having MS disease, thus, according to the data, this allele can be considers as a risk factor for the disease.
There was a meaningful association between TG genotype frequency and MS disease (P = 0.001, OR= 12.65, CI= 4.94 - 32.25), which implied there was 12.65 times higher chance of having disease in the individuals with TG genotype. The G allele was more frequent in the control group compared to the patient group, and there was a meaningful relationship between this allele and the chance of having MS disease (P < 0.001). rs2981573 (1380AG): AA genotype had the highest frequency and GG genotype had the lowest frequency in both patient and healthy groups. There was a meaningful association between AG genotype frequency and MS disease (P = 0.04, OR = 0.14, CI = 0.01 - 1.29), which confirmed this genotype as a protective factor against this disease. The A allele was the most frequent one in both patient and healthy groups, which nullified the possibility of any meaningful association between the A allele frequency and the disease (P = 0.503).
4.1. Conclusion
According to the data analysis, there is a meaningful association between GC genotype frequency and MS disease in rs2243158 GC on IL19 (P = 0.0004), which confirms this genotype as a protective factor for the disease. There is also a meaningful association between the G allele frequency and the chance of MS disease (0.004). There is a meaningful association between AT genotype frequency and MS disease in rs2243168 AT on the same cytokine (P = 0.001), which shows the protective character of this genotype for the disease. A meaningful relationship has also been observed between the A allele frequency and the disease (P < 0.001, OR = 4.73), which makes this allele a risk factor for MS. There is a meaningful association between TG genotype frequency and MS in 1053TG, rs2981572 on IL20 (P < 0.001), which shows this genotype is a protective factor for the disease and there is also a meaningful relationship between the G allele frequency and this disease (P = 0.001). There is a meaningful association between AG genotype frequency and MS in 1380AG, rs2981573 on IL20 (P = 0.04, OR = 0.14), which demonstrates the protective characteristics of this genotype for MS. No meaningful association has been observed or reported between A and G alleles’ frequencies and the MS disease.