1. Context
Infectious disease is one of the leading causes of death globally, leading about 50,000 people to lose their lives daily (1, 2). In recent years, drug resistance in human pathogenic bacteria has been reported worldwide due to the continued use of antibiotics. In addition to this problem, some antibiotics have adverse effects on the host, including severe allergies, immune system suppression, and allergic reactions. This issue has created many clinical problems in treating infectious diseases, so there is a need to develop natural and alternative antimicrobial drugs to treat infectious diseases. Medicinal plants have been studied as natural resources for treating infectious diseases worldwide in recent decades (3-6).
Medicinal plants are valuable natural resources in developed countries and are used as raw materials to produce safe drugs for humans. In this regard, Iran is one of the richest resources for medicinal plants globally, with a high diversity of habitat conditions for these plants (7-9). Medicinal plants as raw materials are considered in producing safe drugs for humans. Pharmacological researchers are looking for 21st-century drugs in plants and believe that they are the solution to medical problems, so many drugs have been extracted from plants as secondary metabolites to treat infections or be used as antioxidant and anticancer agents (3, 10).
Secondary metabolites are organic compounds that, unlike primary metabolites, are not directly involved in the growth, development, and reproduction of an organism. Although the absence of secondary metabolites does not cause immediate death, it can impair survival and fertility or change appearance in the long run, but sometimes causes no change. Plants' secondary metabolites have been used to treat infections and diseases since the beginning of human life. During the past hundred years, synthetic chemical drugs have replaced natural compounds that have been modeled on the structure of plants to make chemical drugs such as aspirin and salicylic acid. Today's growing market demand for renewable natural products and recognizing plants as potential producers of biochemical products have created new research fields for the production of secondary metabolites. Since the second half of the last century, extensive research has been done on medicinal plants in most countries, and thus, many herbal medicines have been prepared and delivered to the market. Iran has a rich flora with more than 7,500 plant species, many of which are medicinal. The necessity of studying effective medicinal substances (antioxidants) has made the natural flora of Iran important (4, 11-13).
Natural citrus and vegetable antioxidants have been shown to inhibit the growth of important pathogens. Some researchers have confirmed the relationship between fruit and vegetable consumption and chronic disease reduction. Although fruits and vegetables are varied in terms of antioxidant compounds and activity, they usually contain antioxidants and thus have high antioxidant activity. On the other hand, antioxidants have other applications in addition to treating and preventing cancer and atherosclerosis; for example, natural antioxidant compounds of olive leaf have been used to increase the storage of fats and oils, and peanut skin extract has been used due to its antioxidant activity to preserve potato chips (14, 15).
Research has shown that the sources of phenols and flavonoids in different parts of the world depend on the diet of the region's people. For example, in the countries such as Japan and China, the consumption of green tea provides these compounds needed by the body, while in Western countries, these substances are supplied by consuming apple and onion, and in Eastern countries, by consuming fermented vegetables and foods. In Iran, there is no specific use of substances containing antioxidants, but with various advertisements, consuming raw and cooked vegetables, leaves of various plants and trees (in the form of herbal tea, essences, essential oils, extracts, jams, syrups, pickles, detergents ingredients including cedar, and even in the form of curdling) have been popular. According to various research, different parts of plants with unique antioxidants should be used for these purposes (16-18).
A balance between free radicals and antioxidants is essential for proper physiological functioning. Free radicals contaminate lipids, proteins, and deoxyribonucleic acid (DNA) and lead to some diseases such as cancer, diabetes, Alzheimer's disease, and Parkinson's disease. Therefore, using an external source of antioxidants helps combat oxidative stress. Recently, synthetic antioxidants such as hydroxytoluene and butyl hydroxyanisole have been reported to endanger human health. Therefore, the search for effective and non-toxic natural compounds with antioxidant activity has increased recently (3).
Among medicinal plants, S. striata has traditionally been used to cure infections and wounds among the Zagros residents. Therefore, this study aimed to evaluate the therapeutic and healing effects of S. striata with an emphasis on oxidative stress and gastric ulcer treatment.
2. Reasons for Creating Gastrointestinal Ulcers
Gastrointestinal ulcers, especially gastric ulcers, can result from increased acid secretion for different reasons, including nonsteroidal anti-inflammatory drugs consumption, alcohol consumption, prolonged starvation, malnutrition habits, and severe and persistent stress (19, 20). According to the World Health Organization report, from every 10 American people, one during his/her lifetime is involved in peptic gastric ulcers, and about 15,000 deaths occur each year due to the consequence of this disease. The economic impact of this disease is significant, amounting to more than 10 billion dollars annually in the United States (21, 22). Nonsteroidal anti-inflammatory drugs are among the high-consumption drugs in the world. Many studies have shown a link between their use and the occurrence of gastric ulcers in Western societies (23, 24). Bleeding and gastric mucosal lesions are the most common complications of these drugs, challenging medical science so that nonsteroidal anti-inflammatory drugs have been known as the second most common cause of peptic ulcer after H. pylori (25). Treatment of gastric ulcers with chemical drugs such as omeprazole, metronidazole, and ranitidine is involved with side effects and problems such as autoimmunity, and there is the probability of lesion returning after stopping treatment. For this purpose, extensive efforts have been conducted to find effective natural and herbal compounds to treat gastric ulcers (26).
Wound healing is an improvement process that originates from the skin and other tissue damage (27). One of the goals of medical science is to heal wounds in a shorter time with fewer side effects. Shortening wound healing is essential to reduce the risk of infection, wound complications, and costs (28, 29).
According to the abovementioned studies, a gastric ulcer can be created in various ways in animals, such as utilizing nonsteroidal anti-inflammatory drugs, ethanol, cold water stress, and broken glass. The side effects of gastric ulcer controlling drugs (histamine antagonists and gastric acid secretion pump inhibitors) and the lack of good reports about the effects of this plant and, on the other hand, the antiseptic and antimicrobial properties of the hydroalcoholic extract of S. striata (30) have been proven. Also, few reports have been provided about the healing effects of S. striata on open wounds of rabbit skin (31), anxious and depressive behaviors of adult male mice (32), oxidative stress-induced neurological behaviors (33), repair of the second-degree burning wound in rats (34), and Candida albicans in vitro (35). Therefore, we decided to study the treatment and healing properties of S. striata with an emphasis on oxidative stress and gastric ulcer treatment.
3. Specifications and Ingredients of Scrophularia striata
Antirrhinum majus is a plant with a local name of S. striata (Figure 1). It is a wildling and perennial plant from the aphid plant family, found in most temperate and tropical regions of Iran, including Ilam and some regions of Khuzestan, Kermanshah, Kurdistan, Lorestan, South Khorasan, Fars, Chaharmahal & Bakhtiari, and Kohkiluyeh & Boyer Ahmad. The chemical composition of this plant has not been identified. However, people living in Ilam province have been using this plant experimentally for many years in various forms such as edible decoction, incense, and poultice for the treatment of various diseases such as inflammation and infection of the eyes and ears, skin burns, infectious wounds, episiotomy, pain, gastrointestinal disorders, colds, hemorrhoids, and boils. Also, this plant not only has a strong effect on disinfecting the gastrointestinal tract but also has other effects, including regulating and lowering blood pressure and treating depression. The branches of this plant are used as a stomach tonic (36).
The compounds such as alkaloids, glycoside resins, iridoids, cryptophilic acid, and flavonoids have been identified in the A. majus plant family (36). Five compounds have been identified in S. striata, including cinnamic acid, three flavonoids (quercetin), isorhamnetin-3-O-rutinoside, nepitrin, and one phenylpropanoid glycoside (acteoside 1) (37).
Some species of S. striata have been used since ancient times in traditional medicine to treat eczema, ulcers, goiter, wounds, cancer, and fistulas. Some of them are boiled in milk to apply a poultice over the abdomen to relieve or reduce abdominal pain, while their aqueous extracts have been used as a bath to relieve rheumatic pain. Scrophulariaceae species are full of iridoid glycosides, mainly ocubin and catalpa. Iridoids represent a large group of cyclopentane- [c] -pyran monoterpenoids, which have been mentioned as the constituents of S. striata. Besides, S. striata owing to iridoids substances, has shown various biological activities, including antimicrobial, antitumor, hemodynamic, chlorite, liver protective, and anti-inflammatory activities. Promising reports have been provided about chemical prevention of skin and lung cancer by genipin (a type of iridoid obtained by hydrolysis of geniposide) (38).
4. Investigation of Scrophularia striata Antimicrobial Properties
Total phenols, total flavonoids, antioxidant activity, and antimicrobial activity of S. striata extracts have been investigated. The antimicrobial screening has shown a positive relationship of total phenolic content with antimicrobial activity but a negative relationship with antioxidant activity (30).
The antimicrobial activity of S. striata extract against various microorganisms was investigated using the microdilution method, showing the higher antimicrobial activity of ethanolic extract of S. striata against Staphylococcus aureus and Staphylococcus saprophyticus than other extracts (aqueous, methanolic, and estonian). Among Gram-positive bacteria, S. saprophyticus had lower minimum inhibitory concentration (MIC) and minimal lethal concentration (MLC) values (1.6 and 3.2 mg/mL, respectively) in the presence of the ethanolic extract of S. striata. The effect of S. striata extracts on pneumonia was described in order of ethanolic extract > methanolic extract > ethyl acetate extract > aqueous extract (30).
5. Investigation of Scrophularia striata Antioxidant Properties
The lowest content of total phenols (24.6544 ± 3.21 µg/mL) has been at the lowest altitude of Badreh city, where phosphorus, potassium, organic carbon, organic matter, nitrogen, acidity, lime, and silt were present in the lowest amounts. However, the antioxidant activity and the total content of phenols had a direct relationship in the two regions of Darehshahr and Badreh, but in Dehloran, there was an inverse and strong relationship (39).
The amount of superoxide dismutases (SOD) and MAD decreased with increasing levels of different treatments of S. striata extract, but the amount of total antioxidant capacity (TAC) increased (40). In similar studies, there was a relationship between a decrease in malondialdehyde (MDA) and an increase in TAC antioxidants (41-43), although some studies reported a decrease in MDA and the lack of significant effect on TAC antioxidants (44). This increase in TAC was attributed to an increase in creatine kinase levels in the blood, followed by an increase in glutathione (GSH) (as a TAC factor) (45, 46). It has also been reported that increasing plasma TAC levels either alters platelet response to acute conditions (47) or improves total plasma antioxidant capacity (48). Location in nature is one of the main factors influencing the quantity and quality of plant substances (39).
6. Investigation of Wound-Healing Properties of Scrophularia striata
The ability of S. striata to heal skin wounds in rats was investigated. It was found that mice treated with S. striata showed a significant reduction in the wound area during the experiment compared to other groups. In addition, treatment with S. striata reduced the number of lymphocytes, increased the number of fibroblasts in the early stages, and enhanced the number of fibrocytes in the following stages of wound healing. Other parameters such as tissue repair alignment, re-epithelialization, epithelial formation, increased maturation of collagen fibers and fibroblasts, and large blood vessels to the size of capillaries showed significant differences compared to the control group. The best wound healing activity was observed at high doses of S. striata. They also concluded that the S. striata extract caused significant wound contraction and accelerated healing, and it may be recommended to treat various wounds in animals and humans (49).
The hydroalcoholic extract of S. striata for treating skin wounds in rabbits was investigated. The average time of complete wound healing in untreated and treated groups was 21 days with aspirin, 16 days with 1% phenytoin ointment, and 18, 17, and 16 days with 2% ointment, 5% ointment, and 10% of S. striata, respectively. In histological examination, the signs of improvement and development of skin tissue were more evident with S. striata extract treatment (31).
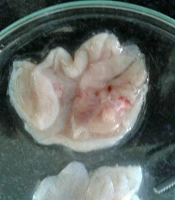
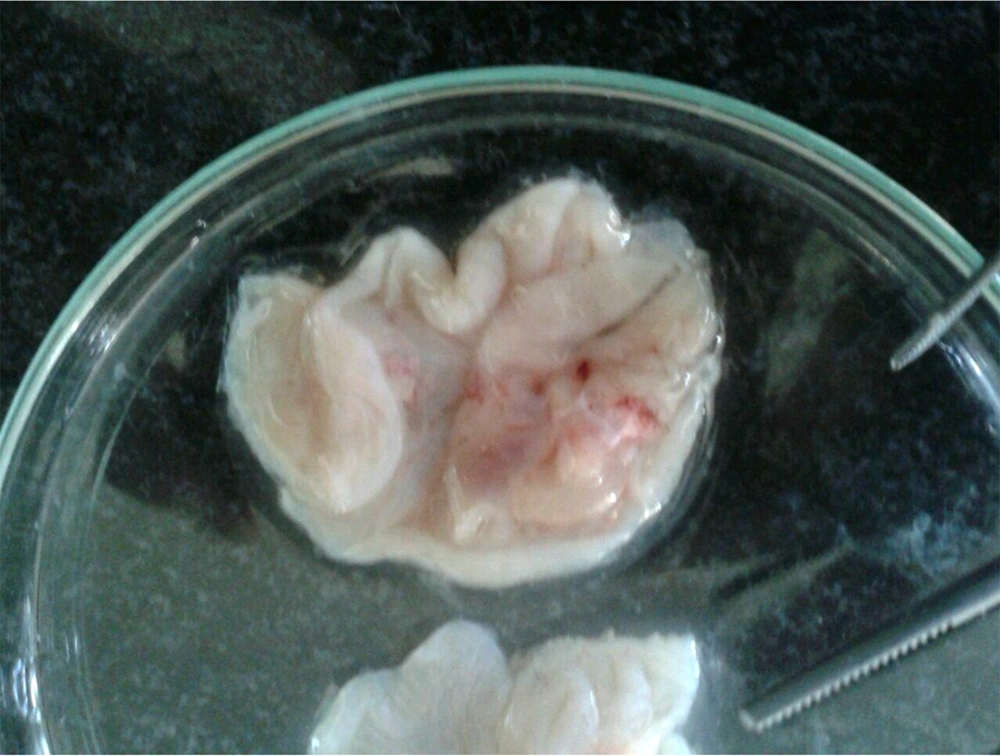
Besides, S. striata extract had a significant positive effect on the size and number of gastric ulcers, and with increasing the concentration of the extract, the size and number of wounds decreased (Figure 2), probably due to anti-inflammatory, antibacterial, and antioxidant activity of its constituents (40). The roots and aerial parts of S. striata have antioxidant properties. The antioxidant and antibacterial properties of S. striata are related to flavonoids, monoterpenes, and coumarin in the extract (50). In a study, the effect of S. striata extract on S. aureus, and Pseudomonas aspergillus was investigated in vitro and its antibacterial effect, equivalent to betadine, was determined (51). Shoohani et al. (31) also concluded that the hydroalcoholic extract of S. striata had an average wound-healing time in the untreated and aspirin-treated groups for 21 days with 1% phenytoin ointment, and 2%, 5%, and 10% of S. striata were 18, 17 and 16 days, respectively. In histological examination, they reported that the symptoms of improvement and development of skin tissue were more evident with S. striata extract treatment. The antioxidant and anti-inflammatory properties of S. striata and the stimulus of fibroblast production in another study (40) confirm the effect of S. striata extract on the healing of rats’ gastric ulcers caused by alcohol.
Internal surfaces of rat stomach due to the effect of Scrophularia striata extract on the treatment of ethanol-induced gastric ulcer (40).
One of the ways to facilitate wound healing is the use of fibroblast growth stimulants. It has been found that increasing the number of fibroblasts in artificial skin leads to wound healing in vitro (52, 53). Oxygen radicals may damage this process with microbial infections (54, 55). Fibroblasts synthesize some components of the primary extracellular matrix of the wound bed, such as fibronectin and proteoglycans, which provide a suitable environment for cell migration and proliferation (56, 57). Fibroblasts then synthesize collagens, which causes stretching in the wound bed (58). Myofibroblasts, which are specific fibroblasts, participate in the process of contraction and wound healing by creating a contractile force (59, 60).
Wound healing has different stages of inflammation, proliferation, and regeneration, each of which comprises several other stages that may overlap and be indistinguishable (61). Therefore, quantitative and qualitative improvement of each stage can accelerate wound healing and reduce complications. On the other hand, the effectiveness of S. striata in the healing process can be due to the presence of iridoid glycoside compounds in different parts of this plant, which inhibit the production of E2 prostaglandin, various interleukins (IL-1α, IL2, and IL-4), tumor necrosis factor and interferon reduce edema and cell infiltration and reduce T lymphocyte proliferation (62, 63). In addition, by increasing the growth of fibroblasts, they provide the basis for more collagen secretion, thereby faster wound-healing (64). In addition, different glycotropenoids in other species of the S. striata family reduce edema, stop cell infiltration, and have anti-inflammatory properties (65). However, phenylpropanoid glycosides inhibit macrophage activity, thus restraining inflammatory chemical mediators' production and ultimately reducing inflammation (66). Besides, phenolic acids with antibacterial properties in some other species of S. striata (67) are another reason for the effectiveness of S. striata in gastric ulcer healing.
During wound healing, an increase in the number of blood vessels, fibroblasts, and epithelial density occurs at the injury site (68). Many studies have been done on the effect of different materials on the stages of tissue healing. Matricaria chamomilla L. extract (69), Camellia Sinensis extract (70, 71), and Aloe vera (72, 73) could accelerate the wound healing process in rats by increasing the number of fibroblasts. The seed extract of S. striata stimulates fibroblasts (74), which is in line with the present study (40), showing the accelerated healing of gastric ulcers caused by alcohol. Hydroalcoholic extract of Portulaca Oleracea L. with antioxidant compounds and anti-inflammatory effects has been influential in the wound healing process of second-degree burns (75). Also, S. striata have compounds with antioxidant and anti-inflammatory properties (68) that confirm the effect of this plant on the healing of gastric ulcers.
The effect of hydroalcoholic extract of S. striata on the prevention of indomethacin-induced gastric ulcers in rats was investigated. The catalase and glutathione peroxidase activity significantly increased in treatments of 50, 100, and 150 mg/kg of S. striata extract compared with the control group. The superoxide dismutase activity of S. striata extract was significantly increased at 100 and 150 mg/kg compared to the control group. Gastric tissue damage in the group receiving 100 and 150 mg/kg of S. striata extract in the group receiving ranitidine and indomethacin was significantly decreased compared to the control group. Besides, S. strata extract increased catalase, glutathione peroxidase, and superoxide dismutase and prevented gastric tissue damage (by increasing endogenous antioxidant levels) (76).
7. Conclusion
Research has shown that S. striata has a positive effect on the size and number of gastric ulcers. Meanwhile, with increasing the concentration of S. striata extract, the size and number of wounds decrease. In general, based on studies, it can be concluded that the S. striata medicinal plant has a significant healing effect on skin and gastrointestinal ulcers, especially gastric ulcers. In addition, further studies are recommended to isolate and identify the compounds of the S. striata extract and show which compounds play the most critical role in the healing of gastric ulcers, but the oral and therapeutic use of S. striata may require clinical tests. The present study investigated only laboratory research results, and none of them was a clinical trial.