1. Background
Pediatric urolithiasis (PU) is one of the most prevalent uronephrological disorders, which is named kidney stones (1, 2). Recently, epidemiological studies have shown that the incidence of urolithiasis is rapidly increasing (3, 4). Changes in dietary habits (i.e., an increase in sodium and fructose intake) are at least partly responsible for the prevalence of PU; nevertheless, the progress made in radiological techniques cannot be underestimated (5). Numerous factors have been shown to increase the risk of developing urolithiasis, including the interaction between genetic and environmental factors, hypercalciuria, alkaline urine, recurrent urinary tract infections (UTIs), and obesity. Moreover, a family history of kidney stones was reported to increase the risk (about threefold) of stone formation (6-9). On the other hand, only a limited percentage of children develop kidney stone disease, even in children with the same environmental exposure. This phenomenon suggests a crucial role of the gene variants in the susceptibility of urolithiasis. Therefore, it would be plausible that understanding the variations of significant genes can gain insight into the etiopathogenesis of urolithiasis to prevent stone recurrence (5).
Approximately 80% of kidney stones (e.g., calcium oxalate or calcium phosphate) contain calcium ions. Previous studies also indicated that calcium is the main component, and calcium oxalate is the most common constituent of kidney stones (10). Therefore, it would be rational to study factors affecting calcium homeostasis in renal tubules. Calcitriol (1,25-dihydroxy vitamin D3) is a type of vitamin D that is active and a steroid hormone that mediates calcium and phosphorus homeostasis and bone mineralization (11). Calcitriol binds to its cognate nuclear receptor, called the vitamin D receptor (VDR). The VDR expression and nuclear activation are necessary events for the functionality of vitamin D, thereby playing a critical role in the emergence of urolithiasis (12). Yao et al. found that under normal conditions, in comparison to wild-type controls, VDR messenger ribonucleic acid (mRNA) levels were lower in the duodenum and higher in the kidney in hereditary genetic hypercalciuric stone-forming rats (13).
Favus et al. demonstrated that the level of VDR was higher (about twofold 49 ± 21 vs. 20 ± 15 fmol/mg protein) in the peripheral blood monocytes of male patients with calcium oxalate kidney stones than their control counterparts (14). In addition, previous studies have shown an association between FokI and BsmI polymorphisms of the vitamin D gene (VDR) with cardiovascular disease and left ventricular hypertrophy. Moreover, the findings showed that different genetic models of TaqI (rs731236), BsmI (rs1544410), and Cdx2 (rs11568820) polymorphisms of functional variants of the VDR gene are linked to a decreased risk of nonHodgkin’s lymphoma (NHL) in the studied population (15, 16). Finally, the aforementioned data showed that VDR might play an important role in urolithiasis pathogenesis. The VDR gene is observed on the 12q12-14 chromosome in humans. In the VDR gene, four single-nucleotide polymorphisms (SNPs) have been discovered up to now, namely ApaI, BsmI, FokI, and TaqI. TaqI polymorphism is defined by a single nucleotide transition (T>C) that results in a synonymous mutation at codon 352 in exon 9, which has been linked to several diseases, including periodontitis, osteoporosis, and multiple sclerosis. Another SNP is FokI which is positioned in the translation initiation codon (12). The relationship between these VDR gene polymorphisms and the risk of urolithiasis has been addressed in numerous studies (17-20). Although most of the previous studies explored the effect of these polymorphisms on urolithiasis in adults, little is known about the role of these SNPs in the development of PU.
2. Objectives
The current study aimed to assess the effect of TaqI gene polymorphisms on the susceptibility to PU in the Iranian population living in Kerman, Iran.
3. Methods
3.1. Study Participants
This case-control study was conducted on 90 outpatients with urinary calculi (49 female and 41 male subjects with a mean age of 4.55 ± 3.005 years) who were referred to the Pediatric Nephrology Clinic in Kerman within January 2016 to March 2018. The diagnosis of urinary calculus was made based on radiopaque stones or children with stone analysis results of calcium-based urolithiasis and further microscopic examinations of urine samples (20, 21). The control group consisted of 90 healthy children who were age- and gender-matched (including 39 female and 51 male subjects with a mean age of 5.6 ± 3.67 years) with no family relationships from Afzalipour Hospital in Kerman. The clinical examinations confirmed the absence of urinary calculus in healthy children. For the purpose of ensuring the absence of urologic diseases (kidney stones) in the control group, the subjects underwent kidney ultrasound, and the presence of hematuria in the urine was assessed by microscopy; accordingly, the possibility of kidney disease (urology) in these patients was rejected. The exclusion criteria of the present research were UTIs, renal failure, chronic and acute kidney diseases, and malignancy for both patients and healthy children. Additionally, the patients with a history of known metabolic, gastrointestinal, hepatic, renal, or endocrinological diseases, noncalcium stones, current use of some medications, obesity (body mass index [BMI]>30 kg/m²), positive urine culture, and anatomical anomalies of the urinary tract were excluded (20, 21). The clinical and demographic characteristics of each subject, including gender, age, height, weight, and family history of urinary calculus, were recorded. Informed consent was obtained from one parent of each participant to authorize the use of biological samples for research purposes. The study was confirmed by the Ethics Committee of Kerman University of Medical Sciences.
3.2. Sample Collection and DNA Extraction
About 2 mL of venous blood was obtained from each subject and stored in EDTA-containing tubes. The leukocyte deoxyribonucleic acid (DNA) was extracted using Prime Prep TM Genomic DNA Isolation Kit (Genet Bio, Korea) according to the manufacturer’s instructions. The quantity and quality of the extracted DNA contents were examined using a NanoPhotometer apparatus.
3.3. Genotyping
Nucleotide changes were determined using polymerase chain reaction (PCR)-restriction fragment length polymorphism analysis for TaqI (rs731236) polymorphism. The amplicons were digested with Taql restriction endonucleases (Thermo Scientific, USA) for rs731236. The PCR temperature cycling conditions were initial denaturation at 94°C for 2 minutes, 30 cycles of denaturation at 94°C for 1 minute, annealing at 60°C for 2 minutes, and elongation at 72°C for 2 minutes. The final cycle was followed by an extension at 72°C for 5 minutes.
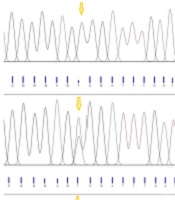
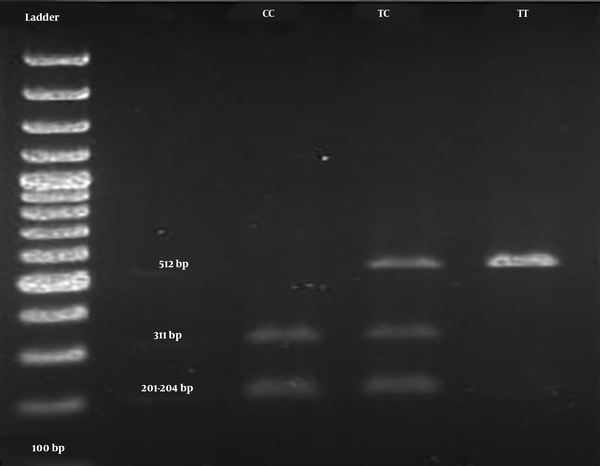
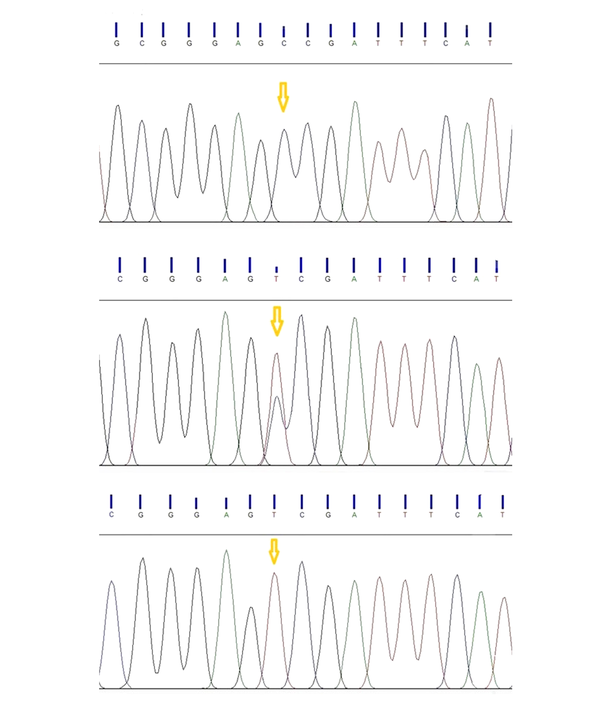
The digested PCR fragments were separated on 2% agarose gels, stained with ethidium bromide (Merck, Germany). Table 1 shows the primer sequences, restriction enzymes, and obtained fragment sizes. Figure 1 illustrates a representative image of PCR products. In this study, the genotypes were confirmed by sequencing 10% of the samples by random selection (Figure 2).
| SNP (Gene) | Primer Sequence (5´→3´) | Primer Length (bp) | Tm (°C) | RE | Amplicon (bp) |
|---|---|---|---|---|---|
| rs731236 (TaqI) | F: 5’-GGGACGATGAGGGATGGACAGAGC-3’ | 24 | 60.3 | Taql | TT 512, TC 512, 311, 201,204, CC 311, 201 - 204 |
| R: 5’-GGAAAGGGGTTAGGTTGGACAGGA-3’ | 24 | 62.0 |
Primer Sequences, Melting Temperature, and Product Sizes for Selected Polymorphism
3.4. Data Analysis
The SPSS software (version 18.0; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Quantitative data are reported as mean ± standard deviation. For quantitative variables, the independent student’s t-test was used to assess the differences between the cases and controls. The chi-square test or Fisher’s exact test was used to compare allele and genotype frequencies between the cases and controls. A logistic regression model was performed to analyze the independent association of rs731236 genotypes with urolithiasis risk by adjusting the age, gender, and BMI. The results were presented as odds ratios (ORs) and 95% confidence intervals (CIs). In the case and control groups, the Hardy-Weinberg equilibrium (HWE) was tested using the chi-square test. The power of the study (α = 0.05) was estimated by the online Power and Sample Size Calculator (biostat.mc.vanderbilt.edu). A two-sided P-value less than 0.05 was considered statistically significant.
4. Results
4.1. Clinical Characteristics of the Study Population
Table 2 shows the frequency distributions of the selected parameters in the case and control groups. Between the patients and controls, there were no significant differences in age, BMI, gender, or urine calcium (P > 0.05). Nevertheless, among 90 children with kidney stones, about 32.3% had a positive family history in comparison to only 3% of healthy children. The difference in urine calcium levels between healthy individuals and patients with kidney stones was statistically significant (P < 0.02).
| Characteristics | Cases (n = 90) | Controls (n = 90) | P-Value b |
|---|---|---|---|
| Age (y) | 4.55 ± 3.005 | 5.6 ± 3.67 | 0.608 |
| Gender | 0.906 | ||
| Male | 41 | 51 | |
| Female | 49 | 39 | |
| Family history | < 0.01 | ||
| Yes | 3 (3.3) | 29 (32.2) | |
| No | 87 (96.6) | 59 (65.5) | |
| Urine calcium (mg/24 hours) | 135.08 ± 20 | 3.58 ± 1.32 | 0.786 |
Demographic and Clinical Data of Infertile Patients and Controls a
4.2. Association between Taql Polymorphisms and Risk of Urinary Calculi
The genotype distributions of the SNP rs731236 were in the HWE in both patient and control groups (Table 3). The main effect models for the TaqI polymorphism (rs731236) in the studied groups are reported in Table 3. Overall, this SNP (rs731236) exhibited a significant association with the risk of PU. Multivariable logistic regression analyses revealed that carriers with the C allele of TaqIrs731236 exhibited a significantly increased risk of PU, compared to those of the control group, even after correction (OR = 1.94; 95% CI = 1.24 - 2.96; P = 0.004). After the Bonferroni correction, the additive and dominant models of TaqI polymorphism revealed an association with an increased urolithiasis risk (additive: OR = 3.10; 95% CI = 1.30 - 6.91; P = 0.011; recessive: OR = 1.98; 95% CI = 0.90 - 4.34; P = 0.112; dominant: OR = 2.42; 95% CI = 1.31 - 4.54; P = 0.007). The TaqI polymorphism had three genotypes in the case and control groups, namely TT, TC, and CC (Figure 1). The frequencies of the three aforementioned genotypes in patients were 30%, 47.78%, and 22.22%, respectively, compared to 52.23%, 35.55%, and 12.22 %, in controls.
| Genotypes/Genetic models/Alleles | Control (n = 90), No. (%) | Case (n = 90), No. (%) | P-Value |
|---|---|---|---|
| TaqI (rs731236) | 0.008 | ||
| TT | 47 (52.23) | 27 (30) | |
| TC | 32 (35.55) | 43 (47.78) | |
| CC | 11 (12.22) | 20 (22.22) | |
| Recessive (TT+TC vs. CC) | 79 (87.77) | 70 (77.77) | 0.080 |
| Dominant (TT vs. TC+CC) | 43 (47.77) | 63 (69.90) | 0.002 |
| Over dominant (TT+CC vs. TC) | 58 (64.44) | 47 (52.22) | 0.097 |
| T | 126 (70) | 97 (54) | - |
| C | 54 (30) | 83 (46) | 0.001 |
| Hardy-Weinberg equilibrium P-value a | 0.145 | 0.714 | |
| Chi-square test | 2.11 | 0.134 |
Association of TaqI Selected Genetic Polymorphism with Hardy-Weinberg Equilibrium of Case and Control Groups
5. Discussion
The VDR gene is 5.6 kb in length and is found on the 12q12-14 chromosome. The VDR gene is linked to calcitriol’s genomic and nongenomic actions in renal tubular cells and modulates the metabolism of citrate, calcium, and phosphate ions (22). Several polymorphisms in the VDR gene have been characterized, among which four SNPs are at the center of attention, namely ApaI, BsmI, TaqI, and FoK I (23). In the present study, the TaqI SNP (rs731236) was assessed; the results showed that it is in the VDR gene and is characterized by a single base transition (T>C) that leads to a synonymous change at codon 352 in exon 9. The four types of polymorphisms of this gene have no considerable difference in terms of the amino acid sequence; however, they have strong linkage disequilibrium. Linkage disequilibrium is the nonrandom correlations of alleles in separate loci and is used to evaluate predisposing genes in complex disorders, such as urolithiasis (24, 25). Previous studies have shown an association between FokI and BsmI polymorphisms of the vitamin D gene (VDR) with cardiovascular disease and left ventricular hypertrophy. Additionally, the findings showed that different genetic models of TaqI (rs731236), BsmI (rs1544410), and Cdx2 (rs11568820) polymorphisms of functional variants of the VDR gene are linked to a decreased risk of NHL in the studied population (25, 26).
The current study provides evidence for the association between the frequency of the C allele and increased risk of urinary stone formation. This finding implies that VDR expression is higher in these patients than in healthy subjects. Moreover, due to the role of VDR in calcium homeostasis, the increased expression of VDR could be ascribed to the formation of urinary stones. The results of the present study are in line with the results of some studies conducted in this area. In a survey carried out by Seyhan et al., they recruited 80 Turkish children with urinary calculi and 40 healthy individuals to assess the impact of vitamin D receptor gene TaqI polymorphism on renal stone formation. Seyhan et al. reported that in patients with the recurrent calcium-stone disease, the frequency of the CC genotype was considerably higher than in the control groups (26).
In addition, in recurrent calcium stone-formers, the C allele frequency was higher than in the control group. It has been revealed that the frequency of the C allele is correlated with the recurrence of urinary calculi (27). In a recent study conducted by Aykan et al., they genotyped a total of 86 first stone-formers, 78 recurrent stone-formers, and 167 controls in the Turkish population and showed that the CC genotype was associated with a higher risk (about threefold) of recurrent urolithiasis (28).
Another meta-analysis study showed a link between the VDRTaqI gene polymorphism and an elevated incidence of urolithiasis when the T allele and CC and TC genotypes were present (29). Therefore, the average and decreased gene expression levels of the VDR gene are associated with the presence of the T and C alleles, respectively. Accordingly, the question is how these variations influence VDR gene expression in the formation of kidney stones. Nishijima et al. (17) reported that the TaqI T allele is associated with an increase in the risk of severe stone disease. Furthermore, Nishijima et al. investigated that increased urinary calcium levels in adult patients with TC and CC genotypes were higher than in those with the TT genotype (17). In another study, Morrison et al. investigated that in patients with homozygous TaqI CC genotype, serum 1,25-dihydroxy vitamin D levels were higher than in individuals with TC or TT genotypes (30). Moreover, Basiri et al. reported that excess uric acid saturation in patients with calcium stone formers with homozygous CC or TT genotypes was similar to this polymorphism but was 67% higher in those with heterozygous alleles (20).
One of the key risk factors for the development of urolithiasis is hypercalciuria. Any physiological or pathological procedures that enhance calcium supply to the urinary system have been proven to increase the risk of stone formation (5). The VDR plays an essential role in the regulation of calcium homeostasis by influencing bone resorption and increasing the absorption of calcium (31). In terms of the mechanism, it is hypothesized that the TaqI variant does not modify the VDR protein structure; nevertheless, it can influence the yield of translation or the stability of mRNA, leading to the alteration of VDR expression and its upstream genes involved in the susceptibility of kidney stone disease (28, 32).
A study conducted by Yamagata et al. (33) demonstrated that in peripheral blood mononuclear cells, the levels of VDR mRNA of the allele C were higher than the allele T. Another study performed by Carling et al. (34) showed that individuals with the genotype CC had significantly higher levels of VDR mRNA. Therefore, individuals with the C allele might be more susceptible to urolithiasis. Of note, several studies have indicated no difference in the distribution of genotypes of the Taql polymorphism between patient and control groups, suggesting that such polymorphisms are correlated with the severity of kidney stone disease (15, 35-38), family history, and recurrence of the disease. These contradictory results might stem from the complexity of urolithiasis etiology, ethical differences, or even different nutritional and environmental factors, such as the use of supplements, hours of exposure to sunlight, and even the economic and cultural levels of patients in different populations. The VDR is an appealing target for developing novel drugs for the treatment of renal diseases. Understanding the precise role of VDR in the kidney can help gain insight into how to deal with renal disorders in future studies.
One of the limitations of this study was the small sample size. As a result, further research with a larger sample size might discover the differences that are statistically significant in this article to be significant in other studies with a larger sample size. In addition, there was no access to the results of blood biochemical investigations and/or 24-hour urine collections. Therefore, in the present study, it was not possible to compare the serum or urine biochemical factors to the reported polymorphism results.
5.1. Conclusions
The current study provides evidence for the association between the frequency of the C allele and increased risk of urinary stone formation of PU in the Iranian population. The polymorphism of TaqI might be a suitable genetic marker for the further study of the possible causes of urolithiasis. Because the present results are preliminary and obtained from a homogeneous group of Iranian patients, the findings should be confirmed. Moreover, the findings of this study demonstrated that C allele (rs731236) and CC variant genotypes were considerably linked with a higher risk of PU in children in the Iranian population.