1. Background
Increased adipose tissue and obesity have a major impact on diabetes and insulin resistance or sensitivity and, therefore, may have significant effect on glucose homeostasis (1). Diabetes (type 1 or 2) is a chronic metabolic or autoimmune disease that is associated with impaired secretion and absorption of insulin. Insulin absorption by receptors may cause several complications such as insulin resistance, decreased pancreatic beta cells, hyperglycemia, etc. (2). Adipose tissue plays a significant role in the production and modulation of energy metabolism (3). Three types of adipose tissue in the human body include white adipose tissue (WAT), brown adipose tissue (BAT), and beige adipose tissue (4).
Beige and BAT have very high metabolism for energy production and stimulation of the mechanism that converts white adipose tissue (WAT) to brown adipose one (BAT). Moreover, beige tissues have been suggested to treat obesity (5). These properties make thermogenic adipose tissue a promising cellular target for treating obesity and diabetes. From among these properties, some important cellular mechanisms have been identified that can be effective in converting WAT into BAT. Beta-adrenergic receptors are very important mechanisms in this regard (6).
Beta-adrenergic receptor-3 (β3-AR) is highly expressed in WAT and BAT, leading to increased lipolysis and thermogenesis (7). Activation of β3-AR has been shown to stimulate lipolysis in WAT adipocytes (8). BAT plays an important role in glucose homeostasis, insulin sensitivity, and metabolism related to the pathogenesis of diabetes (9). One of the regulators of signaling pathway is the control of lipolysis and thermogenesis of the sympathetic nervous system, which activates β3-AR by activating upstream factors. Activation of β3-AR can, in turn, activate lipolysis and thermogenesis factors, one of which is the initiation of the mitogen-activated protein kinase (MAPK) signaling cascade (ERK1/2 activation) (10).
Both β3-AR and ERK pathways have been shown to be involved in the induction of brown adipocytes from white adipocytes in independent ways (11). ERK1/2 is a member of the large MAPK family and includes a family of serine/threonine kinases with cellular and molecular functions (12). In humans and rats, two ERK isoforms (ERK1 and ERK2) are expressed and play role in many cellular processes, including proliferation, inflammation, as well as metabolism (13). According to the evidence already discovered, the ERK pathway is involved in the differentiation of adipocytes, which does it by activating factors such as uncoupling protein1 (CP1) and Peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1α) and enhances physiological functions such as lipolysis and thermogenesis (14).
Increasing physical activity is a useful approach to maintain overall health. When exercising, triglycerides hydrolyzed to free fatty acids are released into the bloodstream and consumed to fuel organs such as muscle. Therefore, regular physical activity results in reduced adipose tissue mass and improvement of metabolism (15). Nonetheless, reducing lipid reservoir is also improved with several other alterations in adipose tissue metabolism, such as changing WAT to BAT, improving mitochondrial function as well as rearrangement of metabolic-associated enzymes (16). In addition to exercising, treatment of some diseases and their complications (e.g., controlling blood sugar and insulin in diabetics, and reducing fat tissue in obese people using herbs or plant extracts) have also been considered around the world. Oral cinnamon plant or its supplement, taken from the stem of an Asian tree called cinnamon osmulfum from lauraceae species, is one of the ancient medicinal plants known to be effective in treating diabetes and lowering blood sugar (17). Cinnamon plays an essential role in regulating glucose metabolism, and cinnamon supplement can reduce serum glucose levels without changing other glycemic parameters and anthropometric indices (18). A clinical study by Lu et al. revealed that cinnamon supplementation improved lipid profile as well as enhanced blood glucose control in people suffering from type 2 diabetes (19). In another study, Bel et al. examined current treatment techniques to reduce WAT and its conversion into beige adipose tissue. A research has shown that exercise as well as β3-AR agonist are currently used as practical method to treat metabolic disorders (20). Wang et al. studied the effect of training and ERK protein gene expression in rat liver tissue and found that aerobic training increased the content and expression of ERK protein gene (21). Many researchers have also argued that the exposure to cold and increased β3-AR expression leads to metabolic improvement (i.e., increased glucose tolerance and insulin sensitivity) (22). The effect of exercise on the regulation of β3-AR and ERK2 protein in visceral adipose tissue has not been properly investigated. However, the interaction of β3-AR and ERK2 protein has been shown to positively affect the regulation of the conversion of WAT into BAT.
Prior to conducting our study, however, the effects of exercise training and dietary supplements (e.g., cinnamon) on these factors and their role in occurrence of thermogenesis were still unknown. Also, the role of changes in temperature affecting the regulation and function of β3-AR and ERK2 protein had not been properly identified. Therefore, further studies were needed to explore these factors involved in occurrence of adipose tissue.
2. Objectives
The current study aimed to investigate the effect of one period of swimming training combined with cinnamon supplementation on β3-AR and ERK2 protein gene expression in the visceral adipose tissue from diabetic rats.
3. Methods
3.1. Subjects
This study was an experimental-fundamental study investigating two experimental and control groups. A total of 35 male Wistar rats, aged two months and weighed between 200 ± 20 g, were selected. Rats were kept in the animal house of Islamic Azad University, Marvdasht Branch at 22 ± 2°C, humidity of 65 - 55%, and dark-light cycle of 12 - 12-hour. The animal food was obtained from the animal house of the Islamic Azad University of Marvdasht and was provided to the animals ad libitum. The required water was also supplied ad libitum in 250 mL bottles for laboratory animals. To induce diabetes in rats, streptozotocin (STZ) solution (dissolved in 0.1 M citrate buffer with pH = 4.5 at a dose of 55 mg/kg body weight) was injected intraperitoneally only once. To ensure that the rats were diabetic, their blood glucose was taken from a blood sample from the tail vein, which was then measured by a glucometer 72 hours after STZ injection. The blood sugar above 300 mg/dL was considered as an indicator of diabetes (23). After induction of diabetes, the rats were randomly assigned to 5 groups, including (1) healthy control, (2) diabetic control, (3) cinnamon supplementation, (4) swimming training, and (5) cinnamon supplementation + swimming training. Seven rats were placed in the healthy control (HC) group to investigate the effects of diabetes induction on the research variables.
3.2. Swimming Training Protocol
The rats were placed in the 36°C pool water in order to test their ability to learn swimming. Then, their activities were observed and recorded for exactly 2 min while they were swimming and attempting to rescue themselves from drowning. The same procedure was repeated three times a week to familiarize them with the training condition. Then, the swimming training was conducted at 36 ± 2°C water. The swimming training was conducted five sessions/week for four weeks. The training time for swimming in the first week was 2 min, to which 30 seconds was added in every subsequent session until it reached 4 min. The swimming was performed in a swimming tank specified for rats with a length of 100 cm and width and depth of 50 cm (24). To prepare an aqueous extract of cinnamon in this study, first 100 grams of cinnamon was obtained from Jihad Keshavarzi of Marvdasht city, and then it was dissolved in 1000 mL of pure water. The solution was boiled for 10 minutes and, after cooling, was passed through paper strainer No. 1. The obtained solution contained 20% aqueous extract of cinnamon which was added to the drinking water of rats so as the daily drinking water of each cage would contain 200 mg/kg cinnamon (there were five rats in each cage) (25). According to the training protocol, training program was not implemented for the control group. The rats also received no insulin therapy during the study period. Regarding the ethical considerations and elimination of the acute training effects and uncontrollable stress variables of the animals during the training protocol, the rats were anesthetized by intraperitoneal injection of a combination of ketamine (30 to 50 mg/kg body weight) and xylazine (3 to 5 mg/kg body weight) 48 h after the last training session. The visceral adipose tissues were then removed from the animals’ bodies, washed in physiological saline, and frozen immediately using nitrogen liquid at -80°C for further evaluations (26).
3.3. Measurement of the Research Variables
3.3.1. Real-Time PCR Analysis
Total tissue RNA was isolated by RNA extraction kit, FavorPrepTM Tissue Total RNA Mini Kit (Taiwan). The quantity and quality of the obtained RNA were assessed by measuring the optical density ratio of 260/280 nm and using NanodropTM spectrophotometer (Nanodrop; Thermo Fisher Scientific, Wilmington, DE, USA) and, then, the RNA was stored at 80°C until cDNA synthesis. The cDNA was synthesized applying 1,000 ng total RNA in a first strand cDNA synthesis reaction and using RevertAidTM First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Reverse transcription quantitative polymerase chain reaction (RT qPCR) was performed using ABI Biosystems StepOne and the RealQ Plus 2x Master Mix Green (Ampliqon A/S, Odense, Denmark). In each reaction, 200 nM of each primer (Table 1) was added to target the specific sequence. Specific primers were designed employing Primer3 software (Table 1). Beta-actin housekeeping gene was also used as internal reference to control qPCR reactions. QPCR conditions were set for 10 min at 94°C followed by 40 cycles of 15 sec at 94°C, 60 sec at 58°C, and final extension of 7 min at 72°C. The amplification signals of different samples were normalized to beta-actin cycle threshold (Ct), and then 2-ΔΔCq method was adopted for comparing mRNA levels of activated vs. control, which were represented as fold change in data analysis (27).
| Gene Name | Primer Sequence | Sizes (bp) | Gene Accession Number |
|---|---|---|---|
| β3-AR | 133 | P26255 | |
| Forward | 5’- TAGCAAGGAGCCTGACTTCTG-3’ | ||
| Reverse | 5’- TTCTGGAGAGTTGCGGTTCC -3’ | ||
| ERK2 | 105 | P63086 | |
| Forward | 5’- ATGGAGCTGGACGACTTACC-3’ | ||
| Reverse | 5’- GCCCTTGTCCTGACCAATTTA-3’ | ||
| Actin, Beta | 122 | P60711 | |
| Forward | 5’-TCTATCCTGGCCTCACTGTC-3’ | ||
| Reverse | 5’-AACGCAGCTCAGTAACAGTCC-3’ |
The Sequence of the Primers Used in the Present Study
3.4. Statistical Analysis
The Kolmogorov-Smirnov test was performed to determine the normality of data distribution. Due to the normality of data distribution in the variables, one-way ANOVA parametric and Tukey’s post hoc tests were utilized. Data analysis was performed using SPSS software (version 23). In the present study, the significance level of the statistical analysis was set at P ≤ 0.05.
4. Results
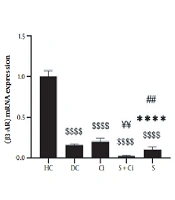
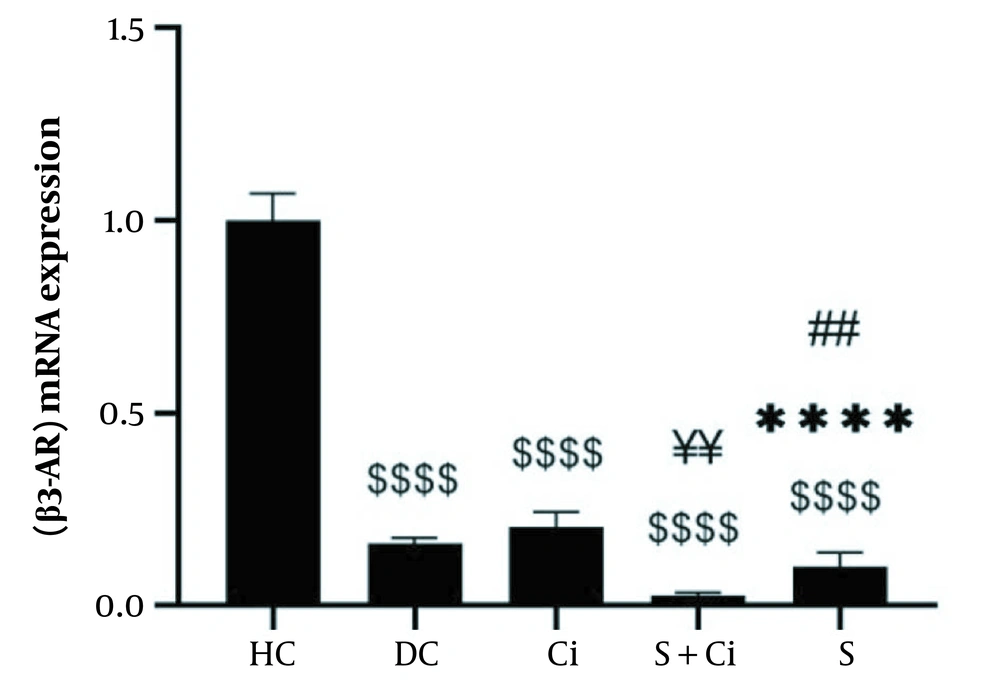
According to the results from the one-way ANOVA test, the levels of β3-AR gene expression were significantly increased in the visceral adipose tissue of diabetic rats after four weeks of swimming training combined with cinnamon consumption (P = 0.0001, F = 11.717). The results of Tukey’s post hoc test revealed no significant difference between the diabetic control group and the Ci (P = 0.092) and S (P = 0.477) groups. Moreover, levels of β3-AR gene expression in visceral adipose tissue in the HC group were significantly higher than those observed in the Ci, DC, S, and S + Ci (P = 0.0001) groups. β3-AR gene expression levels in visceral adipose tissue in the S + Ci group were significantly lower than those in the Ci (P = 0.001) and DC (P = 0.016) groups. In addition, the levels of β3-AR gene expression in visceral adipose tissue in the S group were significantly lower than those in the Ci (P = 0.003) group, though the levels were not significantly different from those in the S + Ci group (P = 0.296) (Figure 1).
Levels of β3-AR gene expression in the visceral adipose tissue of rats in the five study groups ($$$$ P ≥ 0.0001, significant decrease in comparison with the HC group; **** P ≥ 0.0001, significant decrease in comparison with the Ci group; ## P ≥ 0.001, significant decrease in comparison with the C group; ¥¥ P ≥ 0.001, significant decrease in comparison with the Ci; HC, healthy control; DC, diabetic control; Ci, cinnamon supplementation; S, swimming training; and Ci + S, cinnamon supplementation + swimming training).
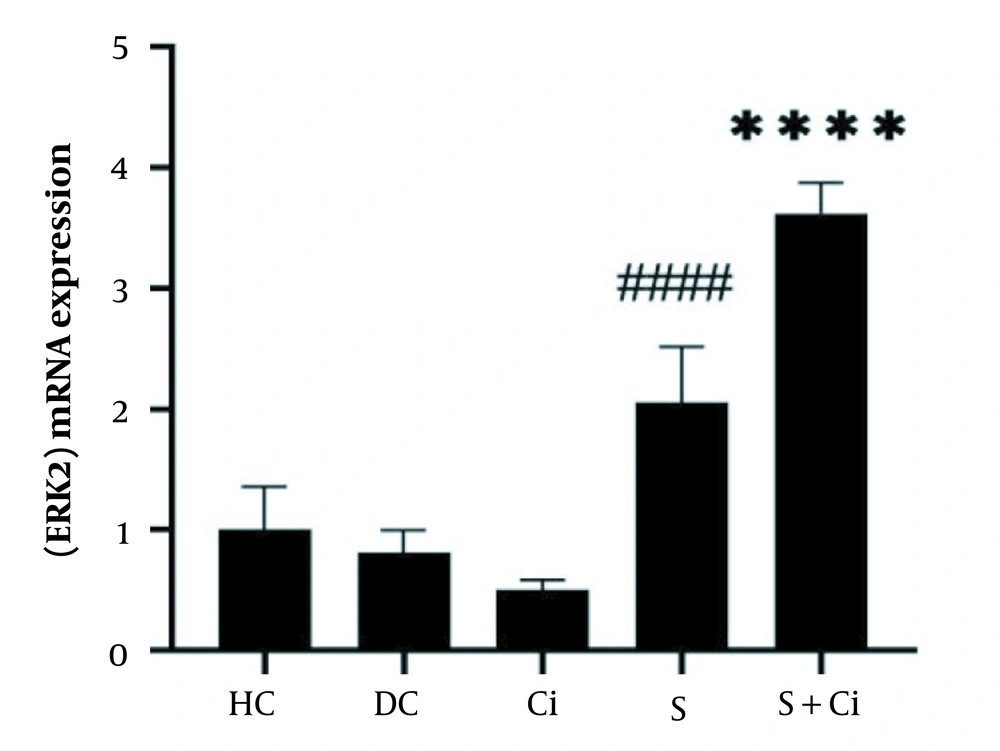
The results from one-way ANOVA indicated that swimming training combined with cinnamon consumption significantly affected ERK2 protein gene expression in the visceral adipose tissue from diabetic rats (P = 0.0001, F = 70.46).
According to the Tukey’s post hoc test results, a significant difference was observed between the S + Ci group and other groups (P = 0.0001); a significant difference was also detected between the S group and the DC and Ci, HD groups (P = 0.0001). In addition, the levels of Β3-AR gene expression in visceral adipose tissue were not significantly different in the Ci and DC groups (P = 0.178) (Figure 2).
Levels of ERK2 gene expression in the visceral adipose tissue of rats in the five study groups (**** P ≥ 0.0001, significant increase in comparison with the HC, DC, Ci, and S groups; #### P ≥ 0.0001, significant increase in comparison with the HC, DC, and Ci groups; HC, healthy control; DC, diabetic control; Ci, cinnamon supplementation; S, swimming training; and Ci + S, cinnamon supplementation + swimming training).
5. Discussion
Analyzing the β3-AR gene expression levels demonstrated that training and cinnamon supplementation significantly affected β3-AR gene expression. Regarding the effect of swimming training, a significant decrease was detected in the swimming training and swimming + cinnamon training groups compared to the control group. In contrast, a significant increase was observed in β3-AR gene expression in the cinnamon consumption groups compared to other groups. Analyzing the data also showed that training and cinnamon supplementation may have significantly affected the expression of ERK2 protein gene. Furthermore, a significant increase was observed in ERK2 gene expression levels due to the effect of swimming training.
As it has been previously reported in the literature, reversing the obesity phenotype by increasing BAT activity is usually recommended for reducing obesity correlation. Training and sports activities have been recognized as a probable intervention to enhance BAT performance. Almeida et al. studied the impact of moderate-intensity training on BAT performance among adult male rats. Training sessions were conducted three times a week with 60% VO2max. Examination of the β3-AR and UCP1 levels showed a significant increase. Accordingly, the researchers suggested that moderate-intensity exercise consistently increased BAT thermogenesis by increasing β3-AR and UCP1 content. This may have been due to the increased activity of the sympathetic system through exercise, which resulted in morphological reconstruction of BAT. Overall, it has been shown that BAT thermogenesis is increased in obese individuals after exercise, and this may lead to a reduction in overall energy expenditure, which may positively contribute to reducing obesity and its associated correlations (28).
The results were inconsistent with those obtained in the study by Almeida et al. (28) on β3-AR. This inconsistency may have been attributed to the fact that the animals in the study were diabetic, which was likely due to impaired insulin production and secretion. In the present study, moreover, no increases were detected in β3-AR levels in the swimming training and swimming + supplementation groups compared with the diabetic control group, while increases were observed in β3-AR levels in the cinnamon supplementation group compared to the diabetic control group.
The main cellular and molecular mechanisms of the conversion of WAT into BATvia β3-AR have been attributed to the sympathetic system. In general, it has been argued that the sympathetic nervous system is one of the main factors of lipolysis and thermogenesis. The secretion of noradrenaline (Na) is bound to β3-AR by the end of the sympathetic nerve. It then activates the cyclic adenosine monophosphate-protein kinase A (cAMP-PKA) axis by binding to the α-subunit of GS proteins (29). The ultimate goal of activating this signaling cascade is to activate hormone-sensitive lipase (HSL) and perilipin, which initiates the lipolysis process in the WAT cells. However, β3-AR signaling in adipocytes can lead to lipolysis by binding to the GI subunit and initiating the ERK1/2-MAP kinase cascade (30). Another role of β3-AR in the BAT adipocytes is to activate UCP1 in mitochondria, which leads to thermogenesis (10). Exercise has been shown to increase adrenergic receptors, especially β3-AR by increasing catecholamines, which can activate the factors such as UCP1 and PGC1α; in fact, thermogenesis is stimulated by increasing the expression of these genes. By increasing BAT and augmenting the innate thermogenesis of BAT, obesity is prevented because lipid and carbohydrate metabolism is increased (31).
In general, exercise therapy can treat diabetes through the improvement of adipose tissue. According to a recent study, aerobic exercises could enhance the secretion of catecholamines, increase the enzymatic activity of hormone-sensitive lipase, and accelerate lipid hydrolysis (32).
In order to activate the MAP-kinase pathway and increase BAT thermogenesis, de Las Heras et al. studied the impact of chronic exercise on factors involved in the regulation of mitochondrial regeneration and biogenesis as well as the ability to generate energy and enhance insulin sensitivity and glucose uptake in BAT of the rats. ERK protein content revealed a significant increase in comparison with the controls. The researchers found that chronic exercise improved mitochondrial function and insulin sensitivity in BAT (33). Their findings were in line with our results regarding the ERK protein increase after doing exercise. In this study, an increase was observed in ERK protein levels in the Ci + S and swimming training group compared to the control group, while the levels in the groups receiving cinnamon supplement were not significantly different from those in the diabetic controls.
Our study was different from the study by de Las Heras et al. (33) in terms of the type of exercise training performed in the study, which was swimming in ours. In our study, swimming was performed in 36°C water, and the results revealed a higher increase in the content of ERK protein in 36°C water than that recorded for the diabetic control group, indicating that training in water may have increased metabolism and fat burning. Although both studies investigated the adipose tissue, the types of studies were different. White visceral fat was examined in the present study, whereas brown fat was explored in the study by de Las Heras et al. (33). It has been discovered that ERK protein plays a role in converting WAT to brown fat employing another protein called irisin (34). Zhang et al. distinguished the signaling transportation pathways of this function from irisin and ERK proteins and showed that irisin protein activated ERK-related signals and had a significant role in converting WAT to BAT (11).
Regarding cinnamon supplementation combined with swimming training, our results suggested that cinnamon supplementation may have been effective in controlling diabetes, blood sugar, and other conditions. In general, cinnamon supplements are among the herbs that play significant roles in the treatment and control of diabetes. Methylhydroxycalcone is an active cinnamon-derived ingredient, which acts like insulin (35).
The sensitivity and phosphorylation of insulin receptors (tyrosine kinase) decrease in individuals with diabetes. Cinnamon stimulates and increases the phosphorylation of insulin receptors and inhibits the enzyme phosphotyrosine phosphatase, which, in turn, increases insulin sensitivity (36). Consumption of cinnamon as food or supplement increases glucose uptake by activating insulin receptors, increasing glycogen synthesis, improving body fat metabolism, and improving the antioxidant status of people with diabetes or metabolic syndrome (37). Furthermore, cinnamon has flavonoid and antioxidant compounds and improves blood glucose and insulin parameters through increasing glucose uptake by various cells in the body and reducing oxidative stress levels (38).
Understanding the increased mechanism of exercise-induced lipid metabolism highlights the importance of exercise therapy in dealing with diabetics. Evidently, diabetes is associated with different degrees of abnormal metabolism of adipose tissue cells and blood lipids (39). Overall, studies have suggested that exercise alters the expression of important metabolic proteins including irisin, ERK, PGC-1α, and UCP1. Some metabolic adaptations to adipose tissue may occur regardless of variations during weight loss. Exercise can play significant role in reducing insulin resistance by maintaining a normal weight and reducing adipose tissue (31).
However, simply measuring β3-AR and ERK2 proteins is not sufficient to evaluate changes in white adipose tissue to brown adipose tissue in response to diabetes and exercise training; seemingly, other proteins including UCP1 α, PGC1, PRDM16, etc. play significant role in occurrence of the thermogenesis process. Regarding thermogenesis, therefore, it was recommended that other cellular mechanisms and the intersection of these pathways with each other should be also considered. In addition, it was suggested that other laboratory methods such as Western blot laboratory method should be implemented in line with the present study’s method (i.e., real-time-PCR), in order to measure both gene expression and protein as well as to compare both methods.
5.1. Conclusions
According to our study findings, cinnamon supplementation and training were capable of changing the expression of β3-AR and ERK2 protein genes. The most encouraging results regarding ERK2 increase, which play a significant role in the regulation of WAT metabolism and can play an important role in the conversion of WAT into brown adipose tissue, were achieved for the group where cinnamon supplement was combined with swimming training. Therefore, it was concluded that cinnamon supplementation may have been extremely effective in regulating adipose tissue metabolism. It was also concluded that a combination of this supplement with training may have been even more effective than single supplementation as a drug. Moreover, the results of the present study revealed that the temperature of the training environment – water temperature (36°C) level, in particular – may have exerted more desirable effect on adipose tissue thermogenesis than the normal ambient temperature. Exercising at a higher temperature may have been an effective stimulant for people prone to obesity and overweight.