1. Background
According to a recent study, with about 1.4 million new cases and 375,000 deaths globally, prostate cancer ranked as the second most frequent disease and the sixth major cause of cancer mortality among males (1). Prostate cancer affects 1 in every 6 men in their lifetime, and the risk increases with age (2). The rate of prostate cancer in Asian countries is substantially lower than that reported by Western countries; however, the literature shows that the rate of prostate cancer is increasing in Asian countries; also, it is on the rise in Iran (3).
In Iran, after cardiovascular diseases and accidents, cancer is the third leading cause of death (4). According to Hassanipour et al.., prostate cancer occurs less frequently in Iran than in other countries. This difference may be due to differences in income, lifestyle, and environmental contexts (5). Prostate cancer is caused by a malignant tumor in the urinary system with biogenetic characteristics (6). Most prostate tumors are adenocarcinomas. Steroid hormones (such as androgens) have an important role in prostate biology. Androgens are involved in developing the prostate and maintaining its function in adulthood. The prolonged presence of androgens seems to be an important cause of prostate cancer. On the other hand, estrogens and androgens appear to be involved in the development of benign prostatic hyperplasia (BPH), though the nature of this role has not yet been elucidated (7).
Experimental data suggest that sex steroids have a role in the development of breast and prostate cancer. The biological activity of sex steroid hormones in target tissues is regulated by several enzymes. If the expression pattern of these enzymes changes, it may modulate intracellular steroid content and play a role in malignant transformation (8).
Two of these enzymes are HSD3B1 and HSD3B2. The HSD3B gene family, located on chromosome 1p13, contains 2 genes and pseudogenes. The HSD3B1 gene (which is expressed in the placenta and tissues such as the breast, skin, and prostate) encodes the 3β-HSD1 enzyme. The HSD3B2 gene (which is most commonly expressed in steroid tissues such as the adrenals, ovaries, and testes) encodes the 3β-HSD2 enzyme (9). These enzymes produce all classes of steroid hormones by catalyzing the oxidative conversion of delta-5-3–beta-hydroxysteroid precursors to delta-4-ketosteroids. The 3β-HSD1 enzyme converts its circulating substrates to downstream steroids (including dihydrotestosterone [DHT], estrogen, and testosterone) in peripheral tissues. 3β-HSD2 synthesizes the steroid precursors needed to produce the male and female sex hormones (cortisol and aldosterone). The 3β-HSD2 isoenzyme, found in the testes, ovaries, and adrenal glands, is responsible for the production of circulating steroids (10). Diseases associated with HSD3B1 include testicular sex cord-stromal neoplasm, lipoid congenital adrenal hyperplasia, and congenital adrenal hyperplasia due to 3-beta-hydroxysteroid dehydrogenase 2 deficiency and 3-beta-hydroxysteroid dehydrogenase deficiency. HSD3B1 and HSD3B play important roles in androgen metabolism and cell proliferation in the prostate. These genes may also be involved in the development of prostate cancer (11). Recent correlational studies have shown a significant association between mutations in region 1p13 (which is the location of HSD3B genes) with prostate cancer. For example, Chang et al. showed that single nucleotide polymorphisms in HSD3B1 and HSD3B2 genes could be associated with an increased risk of prostate cancer (9).
2. Objectives
Due to the importance of this gene in the biochemical pathway of androgens and the relationship of its mutations with prostate cancer, it is expected that the expression of this gene will change. Therefore, in this study, changes in the expression of these 2 genes were evaluated in 2 groups of BPH and prostate adenocarcinoma (PCa) by a real-time method.
3. Methods
3.1. Clinical Sample
Eighty paraffin-embedded blocks were collected from Khatam-al-Anbya Hospital (Tehran, Iran). This study was approved by the Ethics Committee of Islamic Azad University, Tabriz Branch (code: IR.IAU.TABRIZ.REC.1400.178). These 80 samples included 40 PCa and 40 BPH, which were diagnosed between 2020 and 2021. Pathological parameters were collected, including age, pathology stage, Gleason score, and prostate-specific antigen (PSA) range (Sections Sections with 5-µm thickness were prepared from the blocks by microtome. To prevent contamination between samples, the microtome razor was washed with 70% ethanol and DEPC (Diethyl Pyrocarbonate) water. Five sections were prepared from each sample and placed in 1.5ml microtubes. The deparaffinization process was performed by xylene and absolute ethanol according to the previous methods (12).
| Clinical Parameter | Benign Prostatic Hyperplasia | Prostatic Adenocarcinoma | HSD3B1 P-Value | HSD3B2 P-Value |
|---|---|---|---|---|
| Age (mean ± SD) | 55.3 ± 7.3 | 58.6 ± 2.9 | 0.32 | 0.41 |
| Pathology stage | Negative for malignancy | Stage 2: 27 (67.5); Stage 3: 13 (32.5) | 0.84 | 0.59 |
| Gleason score | 0.17 | 0.31 | ||
| ND | 37 (92.5) | - | ||
| 3 + 3 = 6 | 3 (7.5) | 25 (62.5) | ||
| 3 + 4 = 7 | - | 11 (27.5) | ||
| 5 + 5 = 10 | - | 4 (10) | ||
| PSA range | 0.09 | 0.14 | ||
| 10 > | 19 (47.5) | 24 (60) | ||
| 10 ≤ | 31 (77.5) | 16 (40) |
a Values are expressed as No. (%) unless otherwise indicated.
3.2. Gene Expression Analysis
After deparaffinization, 200 μL of initial lysis solution was added to each sample, which included 20 μL of proteinase k (20 mg/µL) and 180 μL of proteinase buffer (Tris-Cl 100 mM, NaCl 200mM, EDTA 2mM, and SDS 1%). The mixture was incubated for 5 hours at 56°C, to prevent RNA degradation, 20 μL of RNAlater was added to each sample After the incubation period, total RNA was isolated using Trizol (Thermo Scientific, USA). The quantity and quality of RNA extracted by spectrophotometer at 230, 260, and 280 nm were evaluated, and after confirming the minimum requirements, the extracted RNA was used for complementary DNA (cDNA) synthesis. Further, 3 μg of RNA was reverse transcribed to cDNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA). A real-time polymerase chain reaction (PCR) assay was then performed with the Rotogene 6000 Real-Time PCR machine (Corbett Bioscience, Australia) using RealQ plus 2X Master Mix Green (Ampliqon, Denmark) in a final reaction volume of 20 µL. Duplicate real-time PCRs were performed for each sample with designed primer sets: HSD3B1 (forward: 5′- CACATGGCCCGCTCCATAC -3′; reverse: 5′-GTGCCGCCGTTTTTCAGATTC -3′), HSD3B2 (forward: 5′- CTTGTGCGTTAAGACCCACAT -3′; reverse: 5′- GGGTTGACTGTAGAGAACTTTCC -3′) and GAPDH (forward: 5′- GTCTCCTCTGACTTCAACAGCG -3′; reverse: 5′- ACCACCCTGTTGCTGTAGCCAA -3). The following real-time PCR conditions were used: 1 cycle of 95°C for 15 minutes, 40 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. In the end, the accuracy of PCR and non-specific amplification was evaluated by analyzing the melting curve for each sample. Expression was quantitated using the 2ΔΔCt method, normalizing to the housekeeping gene GAPDH (13).
3.3. Statistical Analysis
Statistical analysis was performed using SPSS version 26 (SPSS Inc, Chicago, Ill, USA). Further, t test analyses were used to compare differences in quantitative gene expression, and chi-square was used for association analyses. P-values less than 0.05 were considered statistically significant.
4. Results
In this study, changes in HSD3B1 and HSD3B2 gene expression in PCa and BPH were investigated, and their relationships with clinical parameters (including age, stage of pathology, Gleason score, and PSA values) were evaluated (Table 1).
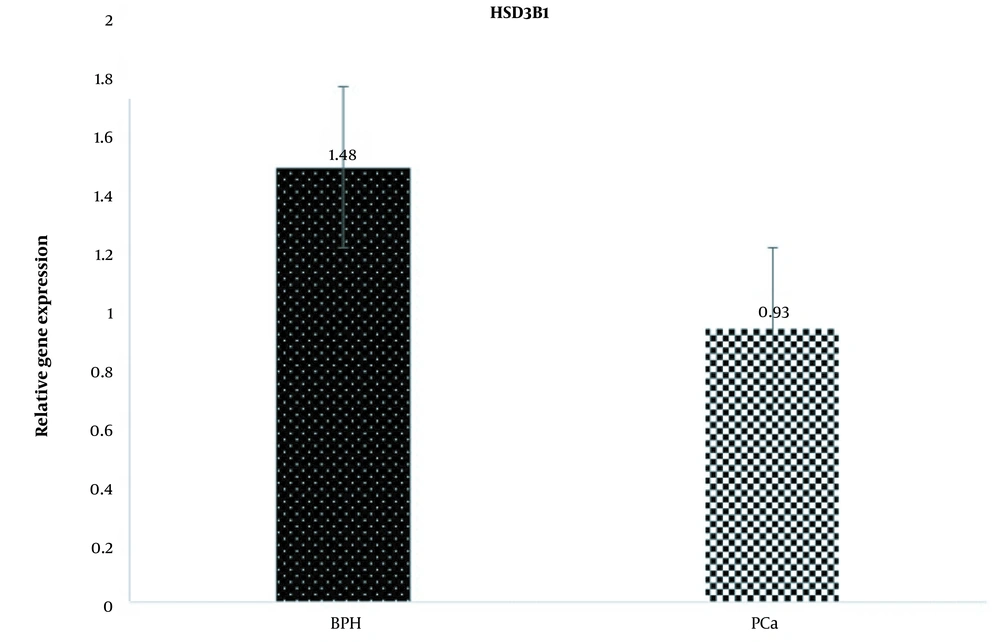
Examination of HSD3B1 gene expression changes between the 2 groups of PCa and BPH showed that the expression of this gene in the PCa group decreased compared to the BPH group (fold change = 0.62). However, this decrease in expression was not statistically significant (P = 0.08; Figure 1).
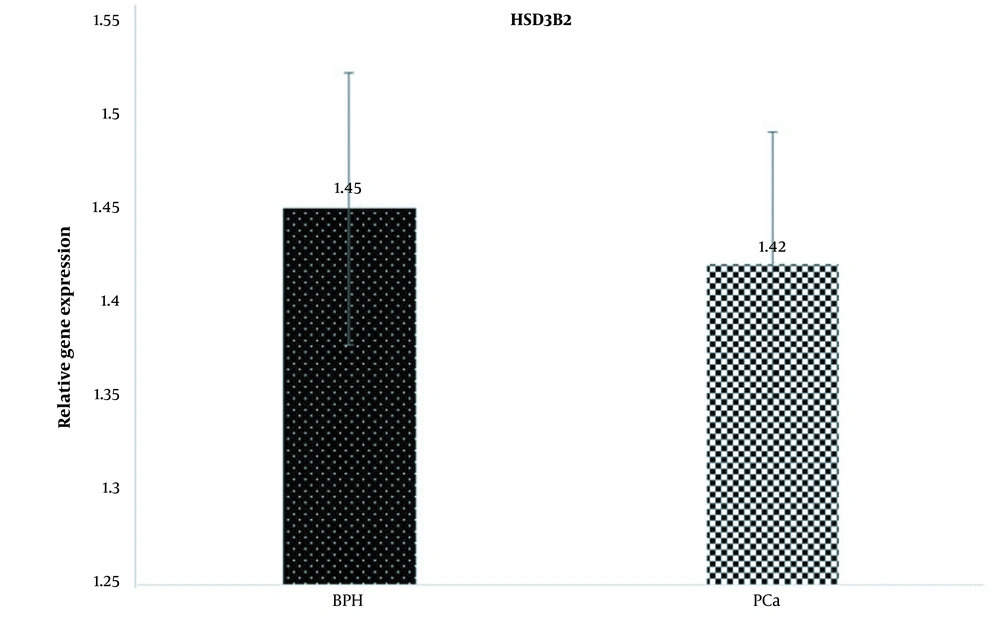
Examination of HSD3B2 gene expression changes between PCa and BPH groups showed that the expression of this gene in the PCa group decreased compared to the BPH group (fold change = 0.97). However, this decrease in expression was not statistically significant (P = 0.14; Figure 2).
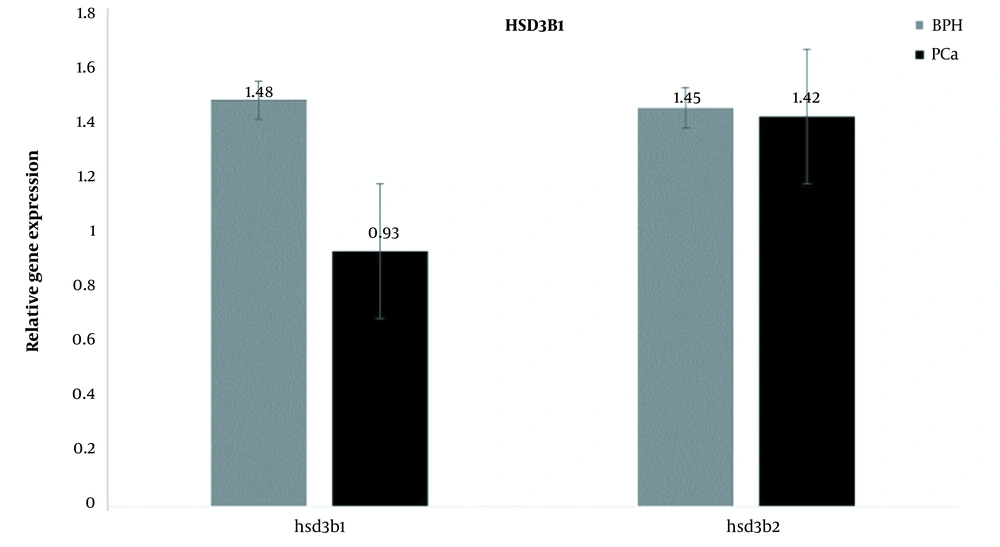
When the expression of 2 genes in 1 group was assessed, the results showed that in the BPH group, the expression of the 2 genes was almost the same, but in the PCa group, the expression of the HSD3B1 gene was lower than that of the HSD3B2 gene (Figure 3).
The relationship between clinical and pathological parameters (including age, disease stage, Gleason score, and PSA range) with the expression of HSD3B1 and HSD3B2 genes in different groups was investigated (P > 0.05).
The mean age of the BPH and PCa groups was 55.3 and 58.6 years, respectively (which showed no significant relationship with the expression changes of the 2 genes with this parameter (P > 0.05).
Among patients with PCa, 27 were in stage 2, and 13 were in stage 3; no significant relationship was observed between changes in the expression of 2 genes and the stage of the disease (P > 0.05).
According to the Gleason score, 7.5% of the population in the BPH group had a score of 6. In the PCa group, 62.5% had a score of 6, 27.5% had a score of 7, and 10% had a score of 10. There was no significant relationship between the Gleason score and expression changes of the 2 genes (P > 0.05).
In the BPH group, 47.5% of the population had PSA < 10, and 77.5% had PSA ≥ 10. In the PCa group, 60% had PSA > 10, and 40% had PSA ≥ 10. In this case, no significant relationship was reported between changes in the expression of 2 genes and PSA levels (P > 0.05; Table 1).
5. Discussion
In this study, we evaluated the expression of HSD3B1 and HSD3B2 genes in the BPH and PCa groups. The results showed that both genes were downregulated in the PCa group compared to the BPH group. Prostate cancer is the fifth leading cause of death and the second most common cancer in men worldwide. It may have no symptoms in the early stages of prostate cancer, often has a period of lethargy, and may require active surveillance (14). Localized prostate cancer usually has a long-term survival, while metastatic prostate cancer is incurable even after intensive treatment (15). Traditionally, prostate cancer can be detected by determining PSA. Elevated PSA levels indicate cancer progression (2 consecutive PSA levels ≥ 0.2 ng/mL) (16). Although these tests are not specific and sensitive, in many cases, they lead to early diagnosis of the disease and prevent many unnecessary biopsies. However, pathological examinations are needed to confirm the diagnosis and differentiate between benign and malignant types. BPH means benign enlargement of the prostate due to cell proliferation in the transitional zone and is associated with age. About 50% of 50-year-old men, 70% of 70-year-old men, and 90% of 80-year-old men are affected to BPH risk. The cause of increased prostate size is the proliferation of prostate cells (17). Several studies have investigated the association between BPH and PCa. For instance, in a recent meta-analysis study, it was shown that there was a significant relationship between BPH and PCa. BPH could lead to escalating risks of PCa (18). Androgens are primarily involved in androgen-dependent processes in androgen-dependent tissues (such as the prostate) in the form of testosterone; nevertheless, in prostate tissue, 5α-reductase catalyzes testosterone, which is synthesized by prostate stromal cells and converted into more potent DHT, performing a vital function in organ development. Various investigations have also observed that the level of DHT in prostate tissue is about 10 - 20 times greater than the level of testosterone in either healthy persons or elderly BPH patients (19). DHT, the most potent androgen, is usually synthesized in the prostate from testosterone secreted by the testis (20). The formation or degradation of 5α-polygons and 5α-androgens (such as dihydroprogesterone and DHT) is catalyzed by 3β-HSD family enzymes (21). Therefore, it can be expected that genetic alteration in these genes can be associated with prostate-related diseases (such as BPH and PCa). For instance, it has been observed that the polymorphism of 1245A>C in the HSD3B1 gene is associated with increased de novo synthesis of androgens and worse outcomes in men treated with androgen-deprivation therapy for metastatic castration-sensitive prostate cancer (22). Because in different cancers, the expression profile of genes undergoes many changes (23). Therefore, in this study, we evaluated the expression of HSD3B1 and HSD3B2 genes in 2 groups of BPH and PCa. For this purpose, the messenger RNA (mRNA) expression of these 2 genes was measured by real-time PCR in 40 PCa tumor specimens and 40 BPH specimens. The results showed that both genes were downregulated in the PCa group compared to the BPH group; this reduction in HSD3B1 gene expression was more noticeable but was not statistically significant. These changes in gene expression were compared with age, pathology stage, Gleason score, and PSA range parameters, but no significant differences were observed in any of the cases. These results are consistent with similar studies; for instance, Khvostova et al. indicated that the expression of the HSD3B1 gene in prostate cancer and BPH samples were examined, showing significant increases in the expression of HSD3B1 genes in benign tissues compared to malignant tissues (24); also, Sakai showed a decrease in the expression of HSD3B1 and HSD3B2 genes in prostate cancer samples compared to BPH. However, in stages II and III of the disease, the expression of these 2 genes was significantly increased (25). In addition to prostate cancer, changes in HSD3B gene expression were reported in other cancers, including breast (26), endometrial (27), adrenal (28), and bladder cancers (29). In adrenal adenoma, a significant relationship was reported between increased HSD gene expression and increased cortisol production (28). In another study, it was reported that in triple-negative breast cancer, the HSD3B1 gene was expressed; the expression of this gene was associated with the disease stage (26). Given that in our study, all samples were in stages II and III, we did not observe any increase in expression. In another study, Neubauer et al. showed that the HSD3B2 protein was expressed in prostate tumor specimens, and with increasing tumor stage, this expression increased (30). HSD3B1 and HSD3B2 convert DHEA into androstenedione, and androstenedione into testosterone, both of which might be the precursors for extra enzymatic steps culminating in testosterone and estrogen production. Estrogens can exert both direct and oblique effects on the prostate. These findings suggest that alterations in the expression of these 2 genes, especially HSD3B1, can be considered a risk factor for BPH. Due to the limited data available in this study, it was not possible to compare the expression changes between different stages; it is suggested that this be considered in future studies. It is also recommended that this study be performed on a larger population. In this study, since expression changes were evaluated only at the transcription (mRNA) level, it is suggested that in future studies, expression changes at the translation (protein) level be examined as well.
5.1. Conclusions
HSD3B gene family plays a crucial role in steroid hormone biosynthesis and is thus of particular interest in hormone-dependent tumors such as prostate cancer. A comparison of the expression of HSD3B1 and HSD3B2 in the benign and adenocarcinoma groups showed a decreased expression in the adenocarcinoma group. Since these 2 genes are in the biochemical pathway of this hormone, it can be concluded that increased expression of these 2 genes is associated with increased levels of DHT in BPH. Given that BPH has recently been shown to be associated with PCa and may increase the risk of adenocarcinoma, it is inferred that it may play a role in the onset of malignancy.