1. Introduction
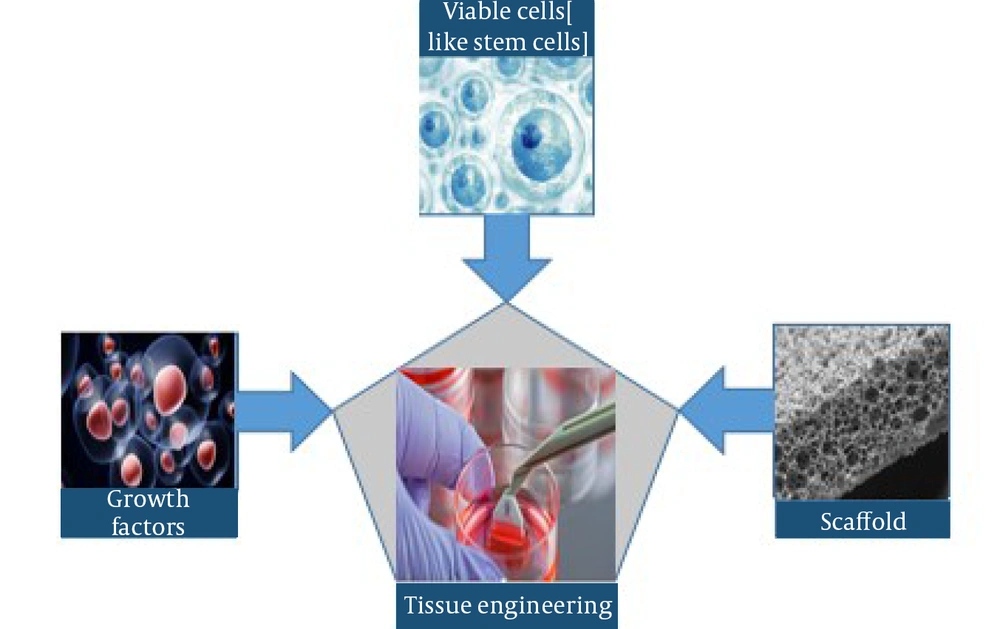
Thousands of people die yearly due to tissue loss or organ failure caused by accidents or diseases. For some patients, tissue or organ transplantation is a vital option. However, this strategy is severely constrained because of a lack of suitable donors (1). Langer and Vacanti described tissue engineering (TE) in the early 1990s as "an interdisciplinary field that utilizes engineering and life science theories to design biological substitutes that repair, conserve, or enhance tissue function" (2). The primary purpose of TE is to stimulate tissue-specific regeneration mechanisms, eliminating the well-known difficulties of organ transplantation (3). Tissue engineering systems apply three essential components to generate new tissues: (1) scaffolds, (2) cells, and proteins such as growth factors, and (3) extracellular matrix (ECM) proteins (4).
The trachea is a flexible cartilaginous tube. In the human body, the trachea serves as a ventilation system for inhalation and exhalation (5). Injuries, inflammation, and innate defects can damage the tracheal cartilage. "Stenting," "suboptimal laser treatment," and "direct anastomosis" are common treatments for injured tracheal tissue, and there are other options, including autografting and allografting. It has also been attempted to use prosthetic materials or a combination of them. However, the results have been unsatisfactory. Tissue engineering is the most recent technology for restoring damaged cartilage tissue (5).
There are growing studies on tracheal regeneration using various cell sources, including pluripotent stem cells (6, 7), Mesenchymal stem cells (MSCs) (8, 9), and terminally differentiated cells (7). Stem cells can change into other types of cells and replace those that have been destroyed. Mesenchymal stem cells also have anti-inflammatory characteristics and can help tissue regeneration (10).
The construction of the proper scaffold is necessary for the production of a functional trachea. The first attempts to reconstruct the trachea were initiated in the late 19th century. Decellularized trachea has been recommended as a suitable scaffold for bioengineered trachea production (7). Tissue engineering is an interdisciplinary field that applies engineering and life science disciplines to maintain, restore, improve, and/or replace damaged body parts. Tissue engineering products are divided into two categories: in vitro and in vivo. It is referred to as an "in vitro tissue-engineered product" when a cell-implanted scaffold construct is placed in a tissue culture plate to regenerate a fully operational tissue. In contrast, in vivo tissue engineering involves placing a cellular or non-cellular scaffold inside a living body to regenerate tissue (11).
2. Tracheal Anatomy and Related Diseases
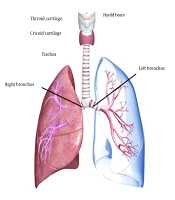
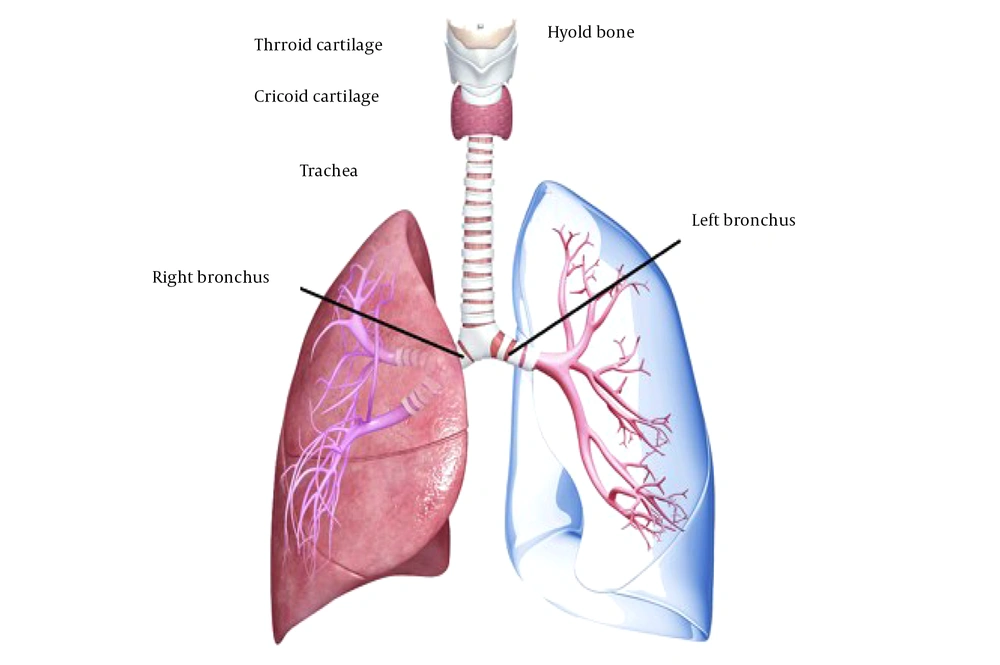
The trachea is a cartilage-fortified tubular channel that connects the upper respiratory tract to the bronchi. It is made up of around 20 imperfect C-formed firm hyaline cartilaginous rings coated with fibroid tissue and supple thews (muscle) (5) (Figure 1). The tracheal wall consists of four layers, which will be explained in the following section. The trachea begins slightly below the larynx, in front of the esophagus, and passes through the center of the chest until it reaches the lungs (12). The trachea is 2.5 cm in diameter and 10 - 16 cm in length. Interestingly, growing evidence suggests that the male trachea is larger than the female trachea (13). However, the etiology of this gender difference has not yet been determined.
2.1. Trachea Wall Cell Composition and Function
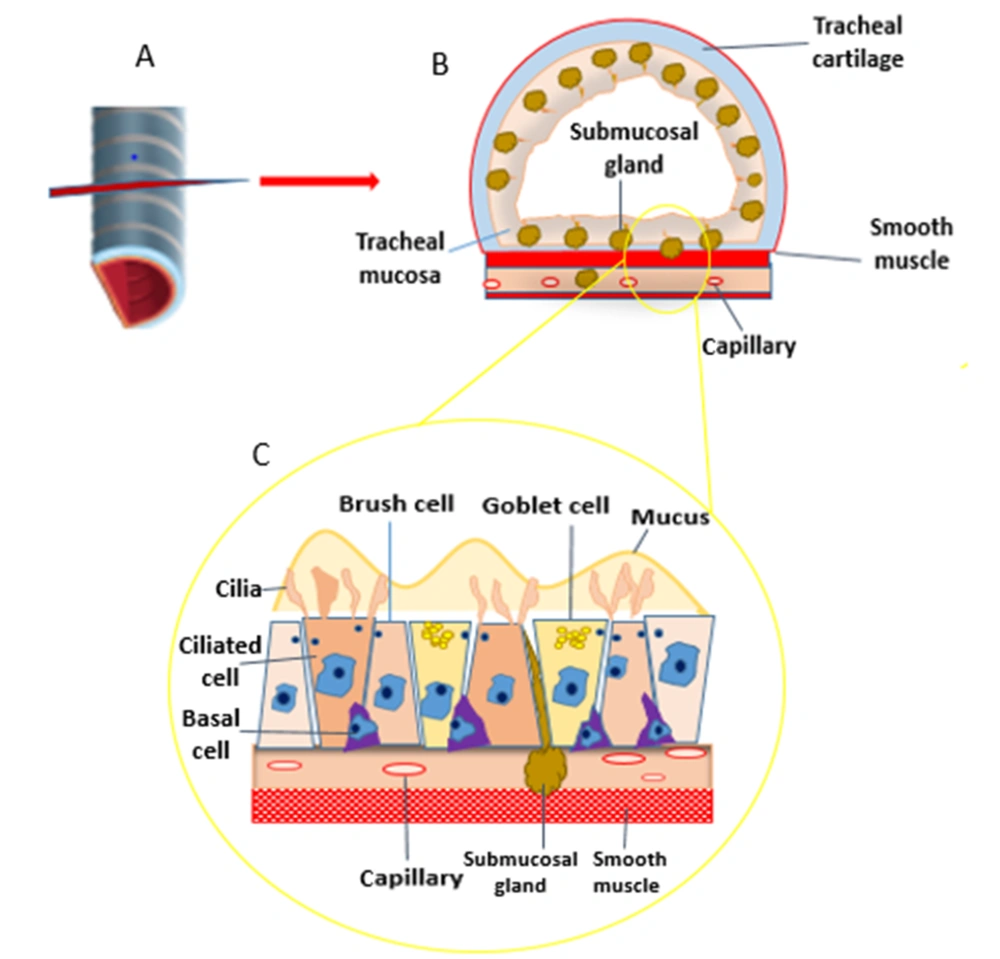
As noted earlier, tracheal tissue comprises four layers with different cell compositions, including mucosa, submucosa, cartilaginous, and adventitia.
- Ciliated cells: They help capture and remove dust and disease particles such as airborne microorganisms.
- Mucus (goblet) cells: They secret mucin that helps moisten the ciliated layer.
- Brush cells: Named tuft cells, they are columnar epithelial cells intercalated between ciliated cells and act as sensory receptors.
- Small granules cells: They stimulate and regulate respiration and vascularization and secrete polypeptide hormones and catecholamines.
- Basal epithelial cells (cells of the basal epithelial layer): They are multipotent stem cells that proliferate to renew the upper epithelial layers whenever needed.
- Fibroblast, plasma, and lymphoid cells: They aid in the restoration of the lamina propria's lowest matrix layer, composed of lymphoid tissue and supple fibers. They assist in epithelial habituation and pathogen particle destruction.
- The submucosa contains fatty and endothelial cells: It is a layer of tissue that lies beneath the mucosa and contains nerves and blood arteries. This layer also contains elastin and collagen fibers, which support and offer elasticity to the trachea. The trachea can change its diameter due to smooth muscle in the submucosa.
The cartilaginous layer contains chondrocytes and aycocytes. Chondrocytes produce a cartilaginous matrix and smooth muscle fibers, which offer flexibility and keep the lumen open (5).
The adventitia is the outermost layer, composed of loose connective tissue that attaches the trachea to the surrounding soft tissues. The trachea can constrict and narrow by the tracheal muscle in the back wall. This movement is beneficial, especially when eating food necessitates esophageal expansion (14, 15) (Figure 2).
There are two forms of tracheal injury and disorders: (1) trachea stenosis, mainly due to injuries, inflammation, tumors, and some inborn abnormalities. When the trachea narrows, it becomes more difficult for air to get into the lungs. Tracheal stenosis can range in severity from mild to severe. Patients with more severe stenosis may require a tracheostomy tube to breathe; (2) tracheomalacia, referred to as damage to cartilaginous walls of the trachea causing weakness or floppiness. Tracheomalacia is a disorder in which the cartilage in the trachea wall softens, resulting in a floppy or weak airway that collapses and makes breathing difficult. A variety of factors can cause tracheomalacia. The disease might be present at birth or emerge later in life (5).
3. Tracheal Tissue Engineering
Tracheal engineering refers to the renascence of the trachea using TE concepts. The "tissue engineering trio" comprises three critical aspects in tissue engineering: Cells, growth factors, and scaffold (16) (Figure 3).
Building a successfully designed trachea begins with a bit of tissue from which cells can be increased to the numbers needed for tracheal tissue formation (17). The mechanical strength of the scaffold is critical for tissue-engineered tracheae because it prevents the airway from collapsing, which could result in major postoperative problems. The composition and architecture of a scaffold determine its mechanical strength (15, 18). A variety of materials have been tried for scaffolding tissue-engineered tracheae. Synthetic biomaterials are typically quite strong mechanically but lack natural biomaterials' bioactivities. The most popular style these days is a mix of natural and synthetic materials (15). The biomaterials and hydrogels used in tracheal engineering must be degradable and can be processed in the liver and/or excreted in the urine (17).
Surgeons can rule out the possibility of death in their patients and enhance patient outcomes by generating a functional tissue-engineered trachea, which existing treatments cannot always do. The application of tissue culture and engineering ideas, as well as a thorough grasp of scaffold features and preparation, can help solve field challenges (17).
4. The Role of Stem Cells in Tracheal Tissue Repair and Regeneration
Stromal or stem cells (MSCs) are popular for cell regeneration in many tissue-engineered organs because they tend to grow into fat, cartilage, bone, and possibly other lineages (16). As immune-privileged cells with potent immunosuppressive capabilities, MSCs may also help prevent tissue-engineered scaffolds from being degraded by host cells (19). Mesenchymal stem cell implantation for tracheal tissue engineering is gaining traction in preclinical studies. Suzuki et al. compared allogeneic rat adipose-derived MSC-soaked collagen sponges to collagen alone to repair anterior tracheal lesions in rats. They found that MSCs increased vascularization and regenerated the epithelium in just two weeks (20).
Chondrocytes, epithelial, endothelial, and smooth muscle cells comprise tracheal tissue. Scholars have also tried to use these cells and other cells to make or repair tracheal tubes. Adult stem cells, Embryonic stem (ES) cells, chondrocytes, induced pluripotent stem (iPS) cells, and other cells generated from a range of giver tissues have been used as cell sources for tissue-engineered tracheal cartilage (5).
Adult stem cells have recently been identified as a potential cell source for tracheal cartilage engineering. Adult MSCs can self-renew, have a long lifespan, and differentiate into several cell types, including adipose, osteogenic, myogenic, and chondrogenic. Bone marrow, adipose tissue, amniotic fluid, the umbilical cord, and other tissues have been found to contain MSCs. Stem cells have proven to be an excellent source of cells for cartilage regeneracy (21).
Previous research has looked at the ability of BMSCs to differentiate into cartilaginous lineages due to various stimuli, including co-culturing with chondrocytes (22, 23), mechanical stress (24), and growth factors (25). The generative and multipotent potential of bone marrow stromal cells was also found to decrease considerably as the donor age increased (26, 27). As a result, finding a better cell source than BMSCs might be necessary. Compared to bone marrow stromal cells, adipose-derived MSCs (ASCs) are less difficult to extract and extend in vitro (22, 23).
In the realm of cartilage engineering, umbilical cord mesenchymal stem cells are also being studied as a new cellular source. Mesenchymal stem cells produced from umbilical cord blood (UCB-MSCs) can be used as a substitute for cartilage tissue engineering (24). Compared to cartilage made from other types of stem cells, amniotic fluid mesenchymal stem cell-derived cartilage is higher in glycosaminoglycan and elastin, making it an excellent cell source for cartilage regeneracy (28). Furthermore, Kunisaki et al. devised a unique method for fetal tracheal repair using AFMSCs as a viable source of cells (29-31). Amniotic fluid mesenchymal stem cells AFMSCs seeded onto synthetic scaffolds create a tissue resembling flexible cartilage more than hyaline cartilage compared to BMSCs and UCB-MSCs (32). Consequently, the best source of mature stem cells for tracheal cartilage engineering should be chosen carefully.
Macchiarini et al. were the first to disclose the trachea created by human tissue implanted with autologous epithelial cells and chondrocytes produced from MSCs (25, 33). In the case of a patient with connate tracheal stenosis, researchers used a decellularized tracheal scaffold with stem cell seeding to substitute an adult airway. After 12 months, they found that the transplant scaffold enhanced revascularization and epithelialization. In a patient struck by tracheal stenosis, stem cells implanted in decellularized human trachea were recently shown to be effective in terms of epithelialization, stableness, chondrocyte production, and neovascularization (34).
5. Types of Scaffolding for Use in Tracheal Tissue Engineering
Scaffolds' primary function is to offer temporary mechanical integrity at the defect site until damaged tissue is healed or regenerated and normal biomechanical performance is restored. In vivo tissue architecture is complicated, allowing cells to interact with one another and the surrounding ECM. The scaffold of a constructed in-vitro model must be built to closely mimic the architecture of the native tissue in-vitro. The selection of the best biomaterial for scaffold construction is an integral part of the model design since it significantly impacts cellular processes. The mechanical qualities of the scaffold should be per those of the tissue to be mimicked in both healthy and pathological states; hence, the material selection is heavily influenced by tissue mechanical properties (3).
Biomaterials are classified as synthetic or natural, and materials are designed to interact with or conform to biological systems used in medical implants. Synthetic polymers have low biocompatibility, leading to unacceptably strong adhesion, and do not closely resemble the ECM (35). When synthetic scaffolds are formed under regulated conditions, properties including porosity, resistance, flexibility, and degradation time can be improved. Scaffolds made of synthetic materials are easier to manufacture in large numbers and endure longer than natural scaffolds (36). Allografts and xenografts are examples of natural polymers. Because of their sources, they have high biocompatibility and cell survival. Nonetheless, they have low mechanical characteristics (28). These components are essential to constructing a sturdy trachea pipe.
Scaffolds made of synthetic hydrolytically degradable polymers include:
- Polylactic acid: PLAs are biocompatible thermoplastics that disintegrate naturally without toxicity in the body. The disintegration rate can be sped up or slowed down by integrating and modifying the lactic acid to glycolic acid ratio (30).
- Polyglycolic acid: Because of its melting temperature of 200°C and the soft, rubbery texture, it changes to between 35 and 40°C (31). Polyglycolic acid (PGA) is a biodegradable polymer used in stitches. It is an ideal polymer to simulate the trachea's flexibility because the human body's temperature is around 37°C. Because of its rapid disintegration rate, it can be used as a temporary scaffold before replacing cartilage cells and epithelial cells. The damaged molecules from PGA, on the other hand, cause the pH to drop, making it difficult for implanted cells to proliferate and hence, reducing viability (32). Furthermore, PGA is less hydrophobic than PLA (31), so an in vivo tissue-created trachea could swell and cause issues.
- Marlex mesh: Monofilament polypropylene mesh is a woven fabric utilized in the chemical craft. It is particularly beneficial because polypropylene is unyielding in the presence of powerful and harsh chemicals (37), which the mesh might interact with when it is produced for 3D scaffolding.
- Polycaprolactone: PCL is widely used for medication transfer due to its modest disintegration speed inside the body and flexibility due to the glass transfer temperature of 54°C (38).
- Polylactide-co-glycolide acid: The monomers PLLA, PLA, or PDLLA are combined by PGA to form PLGA. This copolymer allows for creating a polymer with reduced mechanical, hydrophilic, and/or elasticity defects by selecting a specific ratio. By improving cell proliferation and adhesion, PLGA promotes cell growth and expansion (31).
5.1. Natural Biodegradable Polymer-Based Scaffolds
5.1.1. Polysaccharide Polymers
Sugar chains make up polysaccharides. They are sometimes used in tissue engineering because they are easy to come by and have interchangeable characteristics and biocompatibility (24). Starch, chitosan, alginate, and hyaluronic acid (HA) derivatives like hydrogel are examples of polysaccharide polymers.
5.1.2. Proteins
Proteins make up a significant portion of the human body, making them the most natural scaffolding matter. If this substance is not created correctly, proteases may destroy the protein scaffold enzymatically faster than expected. Furthermore, protein has such low strength, so it cannot be employed as a structure on its own (31). Collagen, serum albumin, silk, and soy are some proteins and poly-amino acids employed for scaffolding.
5.1.3. Biofibers
Biofibers are natural substances derived from wildlife and renewable plants. They are biodegradable and inexpensive to obtain due to their origin. Biofibers have a wide range of applications. They can be used in scaffolds for tissue engineering because of their tiny structure, which enables the adjustment of cell dispersion and pore size (39). A common method for making nanofibers is electrospinning. Synthetic and natural polymers and ceramics can be employed, resulting in scaffolds with adequate surface area to volume ratios for improved diffusion rates (40).
5.1.4. Other Non-biodegradable or Permanent Scaffolds
Some materials used in scaffolds in tissue engineering are resistant to biodegradation or take many years to degrade. This is not the most advantageous quality because it would prevent cells from taking over the scaffold and forming a more native structure instead of operating as a constant scaffold that could be rejected in vivo if not appropriately treated. Glass, stainless steel, polyester polymers, and silicon are some scaffold materials. These materials are more mechanically strong and resistant to change than biodegradable biomaterials, although they do not dissolve (37).
The characteristics of a scaffold used to regenerate tracheal tissue are as follows: (1) there are a lot of holes (90%). Pore diameters range from 5 to 100 m. It improved vascularization, cell proliferation, and pore-to-pore connectivity; (2) the geometry of native tracheal tissue or the form of the tracheal handicap should be replicated by the scaffold; (3) mechanical strength and plasticity are comparable to natural tracheal tissue; and (4) the scaffold should be biodegradable, biocompatible, non-immunogenic, and nontoxic to improve cell survival and avoid graft rejection (32, 40). In tracheal tissue engineering, two primary scaffold methodologies have been proposed: (1) human cadaveric donor tissue that has been decellularized; and (2) synthetic scaffolds produced from scratch. The elimination of all cellular ingredients that are assumed to be capable of evoking an allo-rejection reaction if left behind is the overarching principle underpinning the manufacture and use of cadaveric tissue-derived decellularized biological scaffolds (41).
6. Role of Growth Factors in Tissue Engineering
It is widely assumed that a specific combination of growth factors, scaffolds, and cells will initiate specific interplays among themselves to regenerate a specific tissue. As a result, choosing the right growth factor for a particular tissue (such as the trachea or skin) is crucial (42). Cell proliferation, migration, differentiation, and multicellular morphogenesis are stimulated by growth factors (GFs) during development and tissue healing. Growth factors can help cells expand by supplying them with nutrients (5). They are also essential for tissue repair and regeneration. In the realm of regenerative medicine, recombinant growth factors have attracted a lot of interest (43).
Growth factors are unlike other oligo/polypeptide substances such as hormones and insulin in terms of transfer and reaction. Due to their short half-lives and slow dispersion, growth factors frequently do not work in an endocrine manner; instead, they operate locally after quickly diffusing across the extracellular matrix. The capacity of a growth factor to render a specific message to a subsociety of cells is governed not only by the growth factor's identity and capability to spread through extracellular matrices (ECMs) but also by the number of target cells, receptor types, and intracellular signal transduction following factor binding. A growth factor may send diverse messages depending on the type of receptor and cell to which it is attached. External factors, such as the factors' capacity to attach to ECM, ECM decay, cell target location, and growth factor concentration, can influence a target cell's final response to a specific soluble growth factor (44).
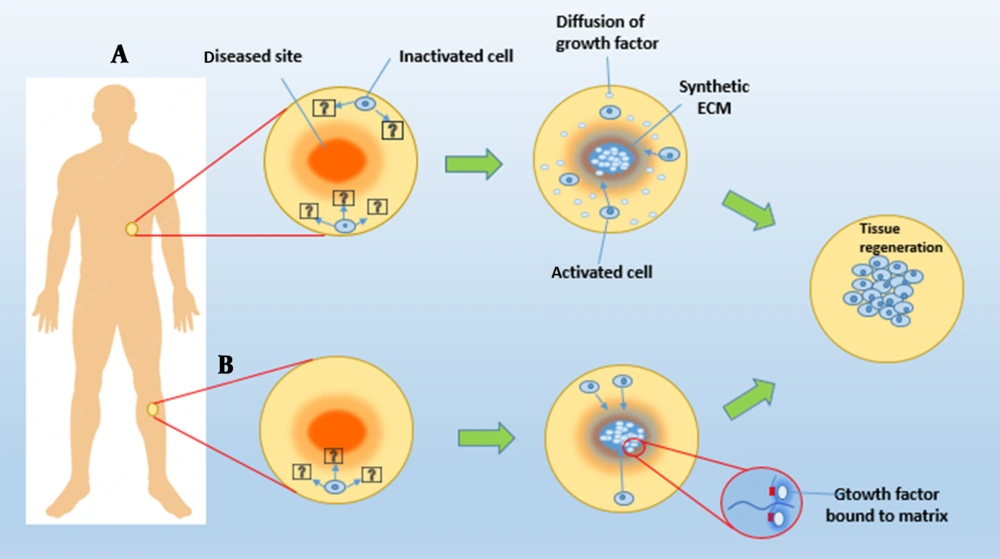
Chemical stabilization of growth factors inside or on the matrix and physical encapsulation of growth factors in the delivery system have been studied as two separate approaches for growth factor biomaterial presentation in tissue engineering (Figure 4). Chemical binding or affinity interactions between a cell or tissue and a growth factor-containing polymer substrate are common in the first technique. In the latter technique, growth factors are encapsulated, diffused, and released in a pre-programmed manner from the substrate into the surrounding tissue. Growth factor patterning on scaffolds in three dimensions can significantly improve factor delivery efficacy (38, 45, 46).
Two tissue engineering methods for delivering growth factors to tissues utilizing synthetic ECMs. A, Synthetic ECMs can guide cell migration and tissue regeneration in specific cell populations by releasing physically enclosed bioactive chemicals; B, Growth factors can also be chemically bonded to a matter system, allowing them to be accessed by the cells that enter it.
Granulocyte colony-stimulating factor, epithelial growth factor, platelet-derived growth factor, insulin-like growth factor, vascular epithelial growth factor, transforming growth factor, and basic fibroblast growth factor (bFGF) have been used for tracheal tissue engineering (TGF) (5).
7. Future Prospects
Building a compound tissue-engineered trachea replacement that is mechanically, physiologically, and functionally equal to the natural trachea will be challenging, and future clinical applications will require appropriate cell sources. Recent developments in tissue engineering have permitted the incorporation of novel procedures to repair tracheal deformations that were previously uncorrectable, thanks to the collaboration of scholars, doctors, medical engineers, and others. Tissue-engineered scaffolds can be used to minimize immunological rejection when donor tissue is not accessible for decellularization. The difficulty of providing these tracheas with the mechanical rigidity to function as needed by human getters persists. As a result, tracheal replacement with a tissue-engineered trachea consisting of biodegradable materials appears to be a more attractive option. However, tissue-engineered constructs still have a long way to go before being largely used and accepted in human clinical experiments.
Since biomaterials such as nano-engineered biomaterials are evolving, a method for fabricating customized tissue-engineered tracheas for each individual will almost certainly exist in the near future. Other issues include improving cell culture and emigration situations on biomaterials, reducing rejection, retaining differentiated tracheal-epithelium cell types, and enhancing epithelial cell survivorship rates and quality following transplantation. As tissue engineering improves, these barriers will be eliminated, and more intriguing innovative ways for future therapeutic applications will be discovered.
8. Conclusion
The tracheal replacement has been offered as a treatment option for patients with end-stage upper airway illness who have failed to respond to present treatments. In tracheal replacement therapy, the use of receptor cells appears to be critical for transplant lifespan, and the addition of foreign cells, once adjusted, may increase graft performance directly. While there is proof that implanting tracheal grafts with autologous cells is generally beneficial, mainly when MSCs are employed, it is yet uncertain which cell types and compositions are most effective in stimulating regeneration.